Chlorine »
PDB 2dch-2dww »
2djg »
Chlorine in PDB 2djg: Re-Determination of the Native Structure of Human Dipeptidyl Peptidase I (Cathepsin C)
Enzymatic activity of Re-Determination of the Native Structure of Human Dipeptidyl Peptidase I (Cathepsin C)
All present enzymatic activity of Re-Determination of the Native Structure of Human Dipeptidyl Peptidase I (Cathepsin C):
3.4.14.1;
3.4.14.1;
Protein crystallography data
The structure of Re-Determination of the Native Structure of Human Dipeptidyl Peptidase I (Cathepsin C), PDB code: 2djg
was solved by
A.Molgaard,
J.Arnau,
C.Lauritzen,
S.Larsen,
G.Petersen,
J.Pedersen,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 24.04 / 2.05 |
| Space group | I 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 87.479, 88.680, 114.350, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.4 / 22.1 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Re-Determination of the Native Structure of Human Dipeptidyl Peptidase I (Cathepsin C)
(pdb code 2djg). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the Re-Determination of the Native Structure of Human Dipeptidyl Peptidase I (Cathepsin C), PDB code: 2djg:
In total only one binding site of Chlorine was determined in the Re-Determination of the Native Structure of Human Dipeptidyl Peptidase I (Cathepsin C), PDB code: 2djg:
Chlorine binding site 1 out of 1 in 2djg
Go back to
Chlorine binding site 1 out
of 1 in the Re-Determination of the Native Structure of Human Dipeptidyl Peptidase I (Cathepsin C)
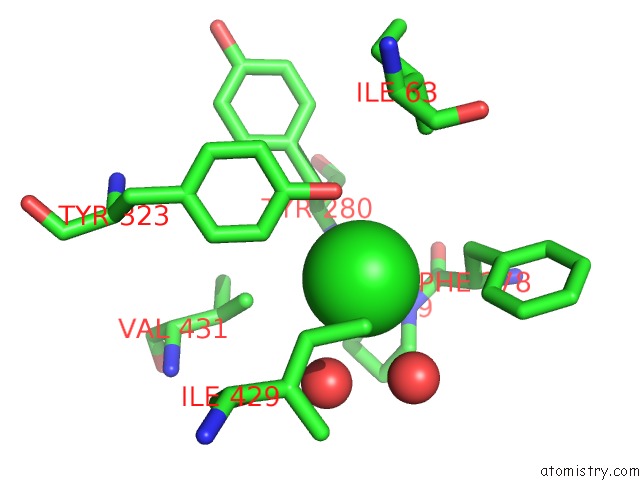
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Re-Determination of the Native Structure of Human Dipeptidyl Peptidase I (Cathepsin C) within 5.0Å range:
|
Reference:
A.Molgaard,
J.Arnau,
C.Lauritzen,
S.Larsen,
G.Petersen,
J.Pedersen.
The Crystal Structure of Human Dipeptidyl Peptidase I (Cathepsin C) in Complex with the Inhibitor Gly-Phe-CHN2 Biochem.J. V. 401 645 2007.
ISSN: ISSN 0264-6021
PubMed: 17020538
DOI: 10.1042/BJ20061389
Page generated: Sat Dec 12 09:03:14 2020
ISSN: ISSN 0264-6021
PubMed: 17020538
DOI: 10.1042/BJ20061389
Last articles
Zn in 8WB0Zn in 8WAX
Zn in 8WAU
Zn in 8WAZ
Zn in 8WAY
Zn in 8WAV
Zn in 8WAW
Zn in 8WAT
Zn in 8W7M
Zn in 8WD3