Chlorine »
PDB 1o5e-1omy »
1o6z »
Chlorine in PDB 1o6z: 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
Enzymatic activity of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
All present enzymatic activity of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form):
1.1.1.37;
1.1.1.37;
Protein crystallography data
The structure of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form), PDB code: 1o6z
was solved by
A.Irimia,
C.Ebel,
D.Madern,
S.B.Richard,
L.W.Cosenza,
G.Zaccai,
F.M.D.Vellieux,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 12.0 / 1.95 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 126.640, 114.420, 124.870, 90.00, 93.11, 90.00 |
| R / Rfree (%) | 18.991 / 26.328 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
(pdb code 1o6z). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 8 binding sites of Chlorine where determined in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form), PDB code: 1o6z:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Chlorine where determined in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form), PDB code: 1o6z:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Chlorine binding site 1 out of 8 in 1o6z
Go back to
Chlorine binding site 1 out
of 8 in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
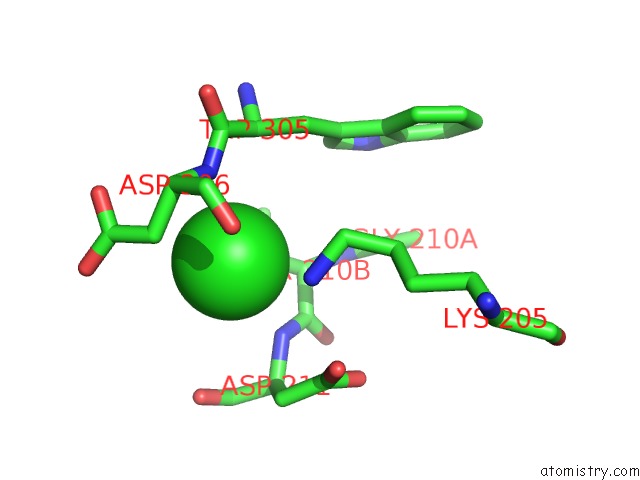
Mono view
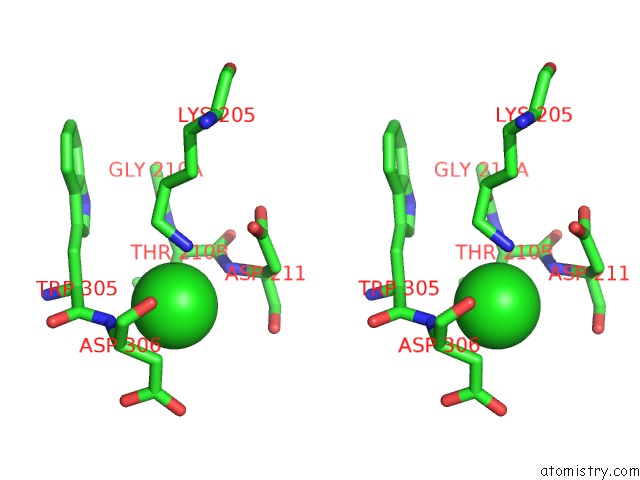
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form) within 5.0Å range:
|
Chlorine binding site 2 out of 8 in 1o6z
Go back to
Chlorine binding site 2 out
of 8 in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
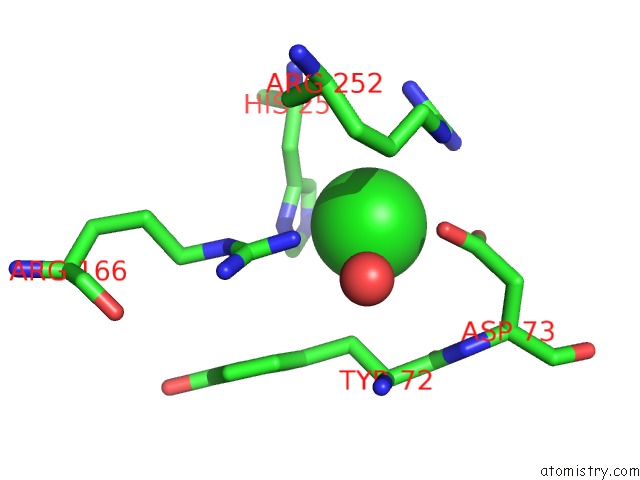
Mono view
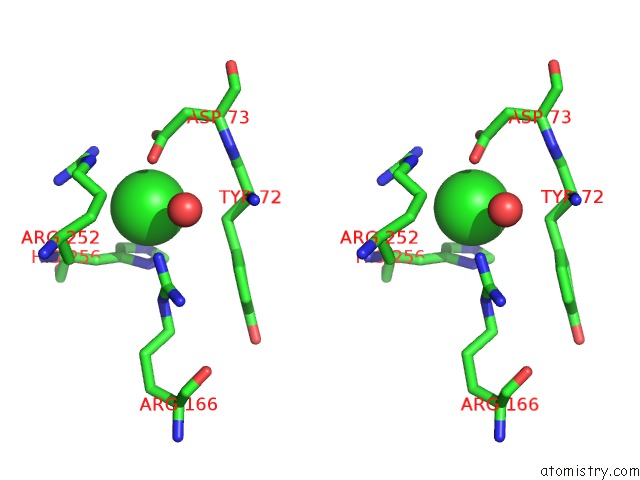
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form) within 5.0Å range:
|
Chlorine binding site 3 out of 8 in 1o6z
Go back to
Chlorine binding site 3 out
of 8 in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
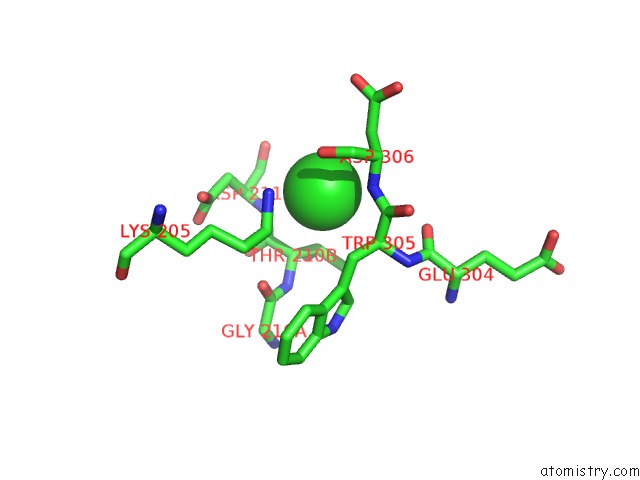
Mono view
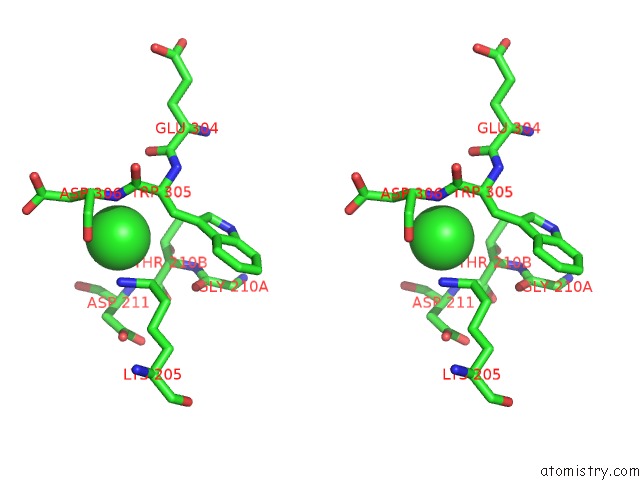
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 3 of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form) within 5.0Å range:
|
Chlorine binding site 4 out of 8 in 1o6z
Go back to
Chlorine binding site 4 out
of 8 in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
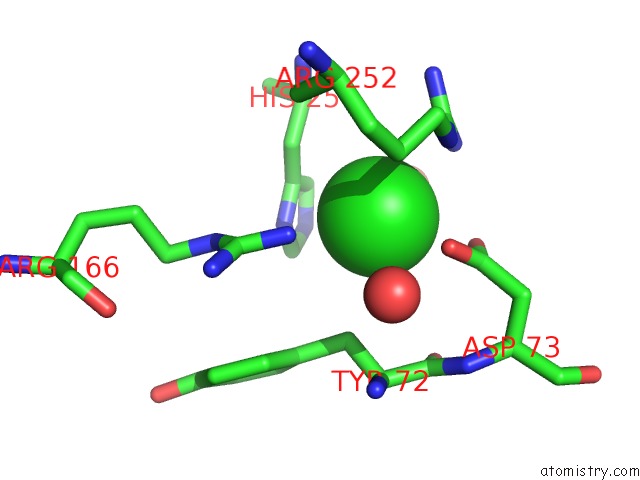
Mono view
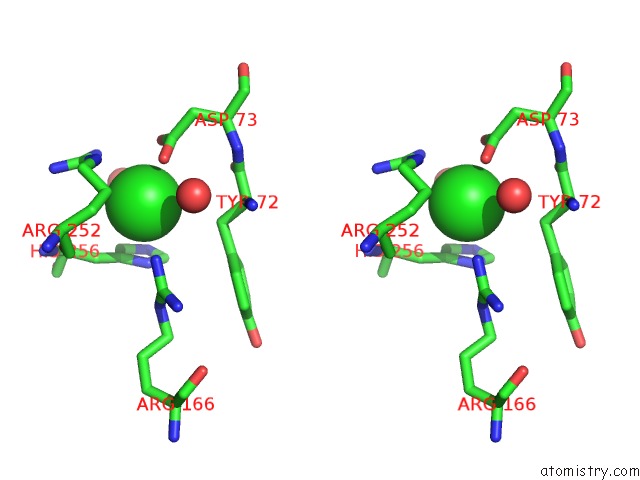
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 4 of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form) within 5.0Å range:
|
Chlorine binding site 5 out of 8 in 1o6z
Go back to
Chlorine binding site 5 out
of 8 in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
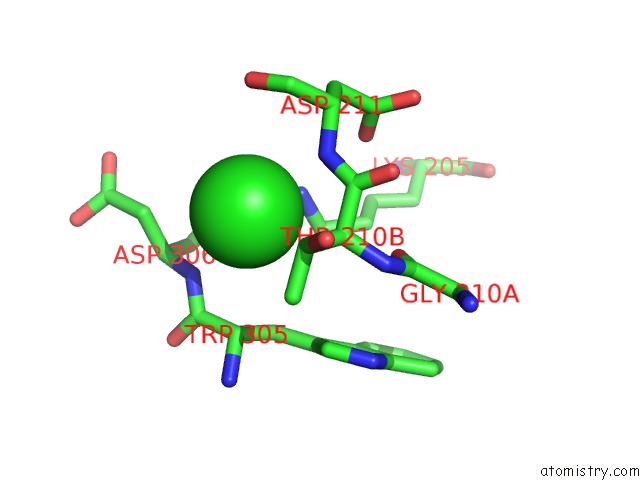
Mono view
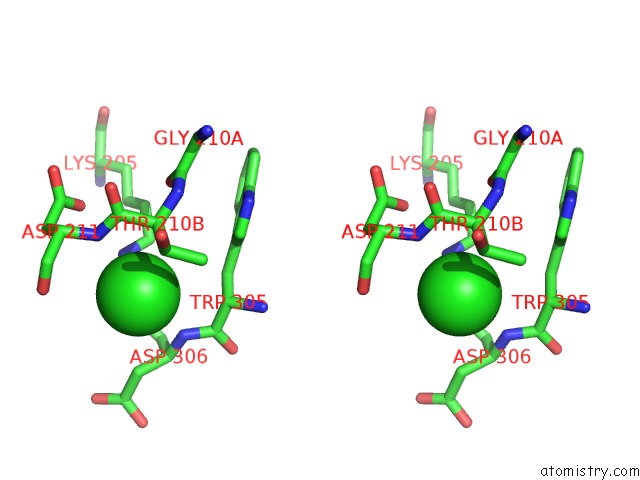
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 5 of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form) within 5.0Å range:
|
Chlorine binding site 6 out of 8 in 1o6z
Go back to
Chlorine binding site 6 out
of 8 in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
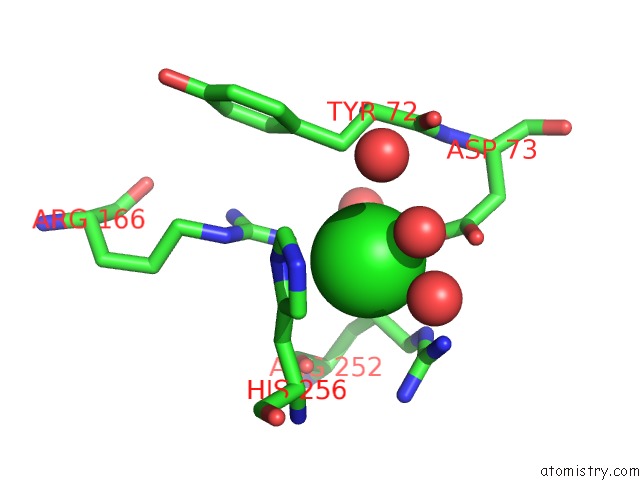
Mono view
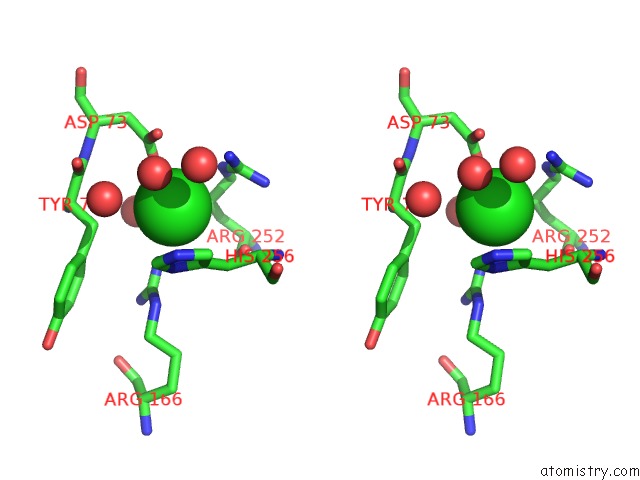
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 6 of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form) within 5.0Å range:
|
Chlorine binding site 7 out of 8 in 1o6z
Go back to
Chlorine binding site 7 out
of 8 in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
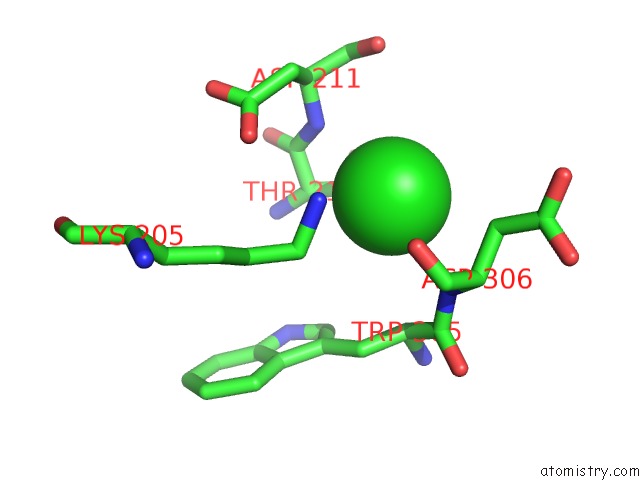
Mono view
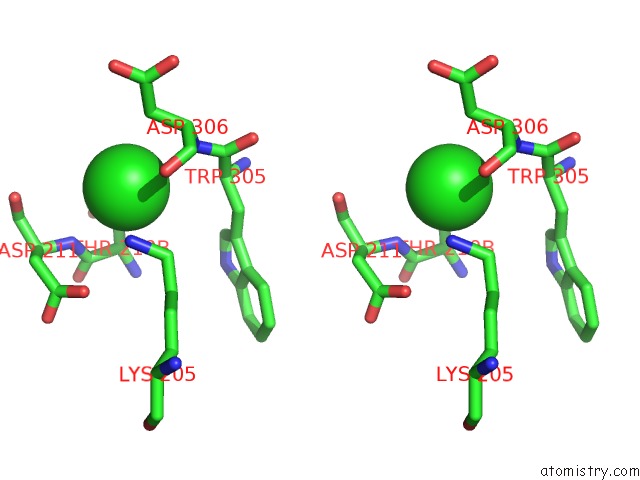
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 7 of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form) within 5.0Å range:
|
Chlorine binding site 8 out of 8 in 1o6z
Go back to
Chlorine binding site 8 out
of 8 in the 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form)
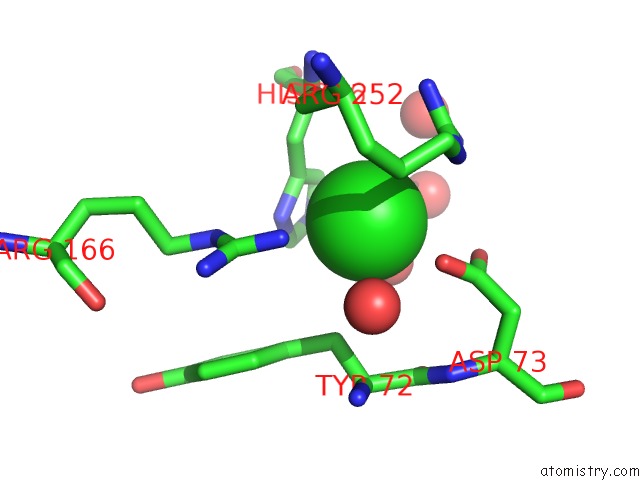
Mono view
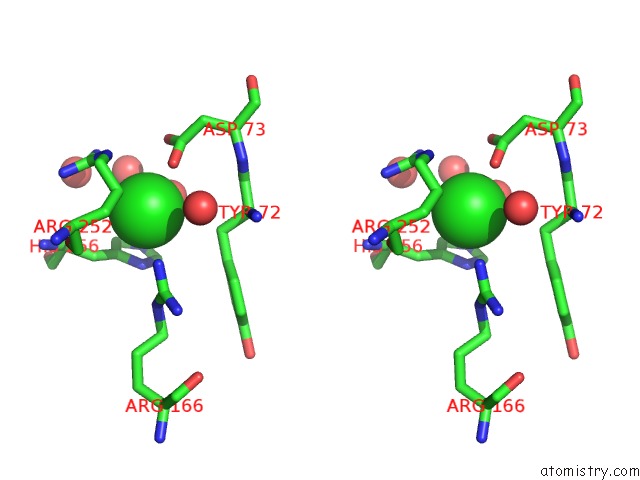
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 8 of 1.95 A Resolution Structure of (R207S,R292S) Mutant of Malate Dehydrogenase From the Halophilic Archaeon Haloarcula Marismortui (Holo Form) within 5.0Å range:
|
Reference:
A.Irimia,
C.Ebel,
D.Madern,
S.B.Richard,
L.W.Cosenza,
G.Zaccai,
F.M.D.Vellieux.
The Oligomeric States of Haloarcula Marismortui Malate Dehydrogenase Are Modulated By Solvent Components As Shown By Crystallographic and Biochemical Studies J.Mol.Biol. V. 326 859 2003.
ISSN: ISSN 0022-2836
PubMed: 12581646
DOI: 10.1016/S0022-2836(02)01450-X
Page generated: Thu Jul 10 18:37:51 2025
ISSN: ISSN 0022-2836
PubMed: 12581646
DOI: 10.1016/S0022-2836(02)01450-X
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO