Chlorine »
PDB 1rky-1s8f »
1ry9 »
Chlorine in PDB 1ry9: SPA15, A Type III Secretion Chaperone From Shigella Flexneri
Protein crystallography data
The structure of SPA15, A Type III Secretion Chaperone From Shigella Flexneri, PDB code: 1ry9
was solved by
A.Van Eerde,
C.Hamiaux,
J.Perez,
C.Parsot,
B.W.Dijkstra,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 43.85 / 1.82 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 50.878, 87.682, 58.703, 90.00, 90.77, 90.00 |
| R / Rfree (%) | 18.1 / 21.1 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the SPA15, A Type III Secretion Chaperone From Shigella Flexneri
(pdb code 1ry9). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 6 binding sites of Chlorine where determined in the SPA15, A Type III Secretion Chaperone From Shigella Flexneri, PDB code: 1ry9:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Chlorine where determined in the SPA15, A Type III Secretion Chaperone From Shigella Flexneri, PDB code: 1ry9:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5; 6;
Chlorine binding site 1 out of 6 in 1ry9
Go back to
Chlorine binding site 1 out
of 6 in the SPA15, A Type III Secretion Chaperone From Shigella Flexneri
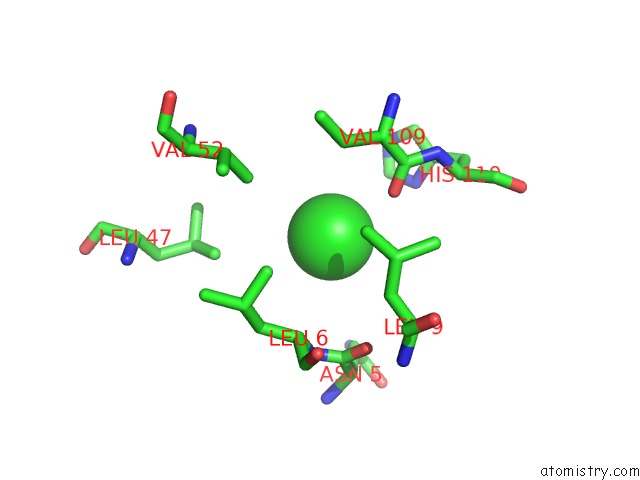
Mono view
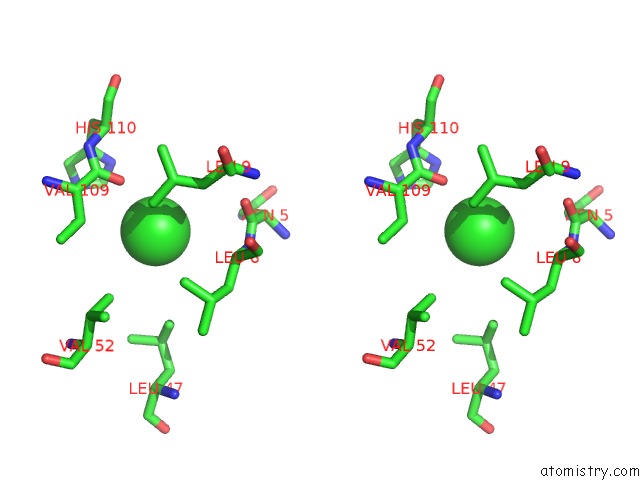
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of SPA15, A Type III Secretion Chaperone From Shigella Flexneri within 5.0Å range:
|
Chlorine binding site 2 out of 6 in 1ry9
Go back to
Chlorine binding site 2 out
of 6 in the SPA15, A Type III Secretion Chaperone From Shigella Flexneri

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of SPA15, A Type III Secretion Chaperone From Shigella Flexneri within 5.0Å range:
|
Chlorine binding site 3 out of 6 in 1ry9
Go back to
Chlorine binding site 3 out
of 6 in the SPA15, A Type III Secretion Chaperone From Shigella Flexneri

Mono view
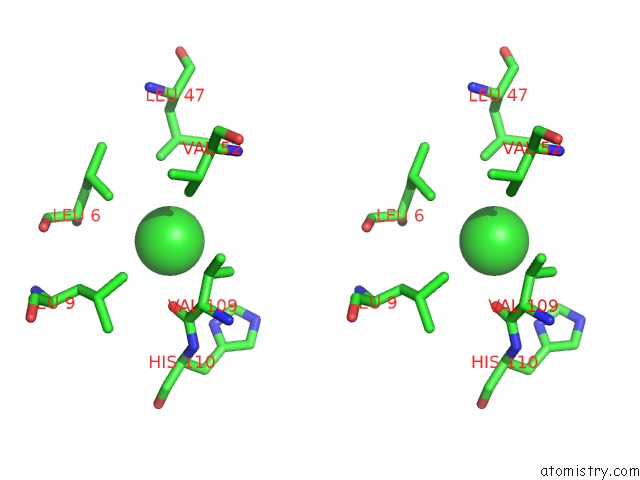
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 3 of SPA15, A Type III Secretion Chaperone From Shigella Flexneri within 5.0Å range:
|
Chlorine binding site 4 out of 6 in 1ry9
Go back to
Chlorine binding site 4 out
of 6 in the SPA15, A Type III Secretion Chaperone From Shigella Flexneri

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 4 of SPA15, A Type III Secretion Chaperone From Shigella Flexneri within 5.0Å range:
|
Chlorine binding site 5 out of 6 in 1ry9
Go back to
Chlorine binding site 5 out
of 6 in the SPA15, A Type III Secretion Chaperone From Shigella Flexneri

Mono view
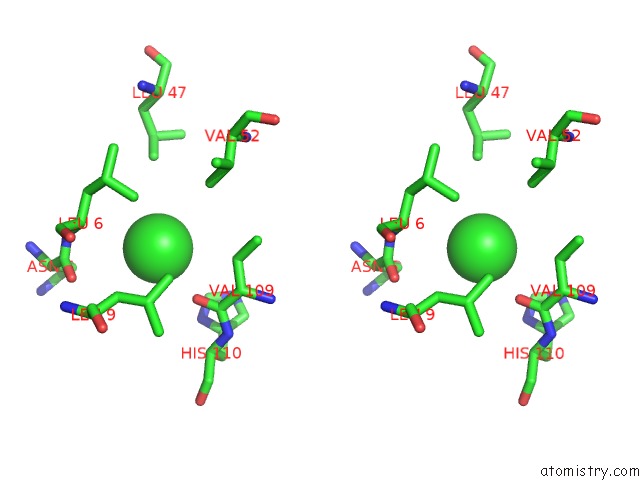
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 5 of SPA15, A Type III Secretion Chaperone From Shigella Flexneri within 5.0Å range:
|
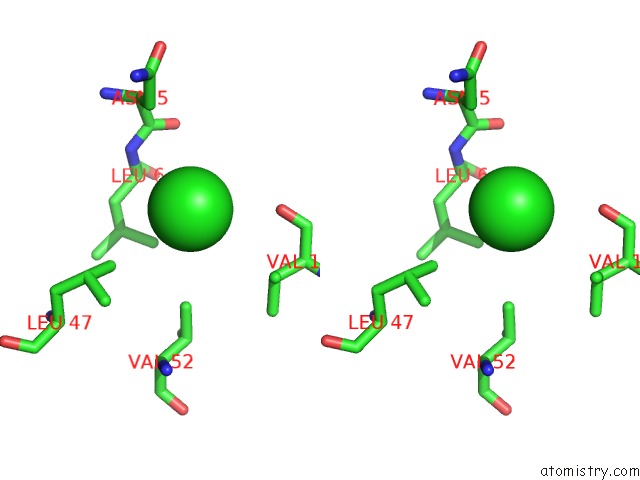
Chlorine binding site 6 out of 6 in 1ry9
Go back to
Chlorine binding site 6 out
of 6 in the SPA15, A Type III Secretion Chaperone From Shigella Flexneri

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 6 of SPA15, A Type III Secretion Chaperone From Shigella Flexneri within 5.0Å range:
|
Reference:
A.Van Eerde,
C.Hamiaux,
J.Perez,
C.Parsot,
B.W.Dijkstra.
Structure of SPA15, A Type III Secretion Chaperone From Shigella Flexneri with Broad Specificity. Embo Rep. V. 5 477 2004.
ISSN: ISSN 1469-221X
PubMed: 15088068
DOI: 10.1038/SJ.EMBOR.7400144
Page generated: Thu Jul 10 19:18:47 2025
ISSN: ISSN 1469-221X
PubMed: 15088068
DOI: 10.1038/SJ.EMBOR.7400144
Last articles
Cl in 8BN6Cl in 8BN2
Cl in 8BNY
Cl in 8BMZ
Cl in 8BN0
Cl in 8BLJ
Cl in 8BMY
Cl in 8BM2
Cl in 8BM5
Cl in 8BK5