Chlorine »
PDB 1swz-1tgx »
1te3 »
Chlorine in PDB 1te3: Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II
Enzymatic activity of Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II
All present enzymatic activity of Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II:
4.2.1.1;
4.2.1.1;
Protein crystallography data
The structure of Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II, PDB code: 1te3
was solved by
Z.Fisher,
J.A.Hernandez Prada,
C.K.Tu,
D.Duda,
C.Yoshioka,
H.An,
L.Govindasamy,
D.N.Silverman,
R.Mckenna,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.00 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 42.703, 41.690, 72.901, 90.00, 104.56, 90.00 |
| R / Rfree (%) | 12.8 / 20.1 |
Other elements in 1te3:
The structure of Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II also contains other interesting chemical elements:
| Zinc | (Zn) | 1 atom |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II
(pdb code 1te3). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II, PDB code: 1te3:
In total only one binding site of Chlorine was determined in the Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II, PDB code: 1te3:
Chlorine binding site 1 out of 1 in 1te3
Go back to
Chlorine binding site 1 out
of 1 in the Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II
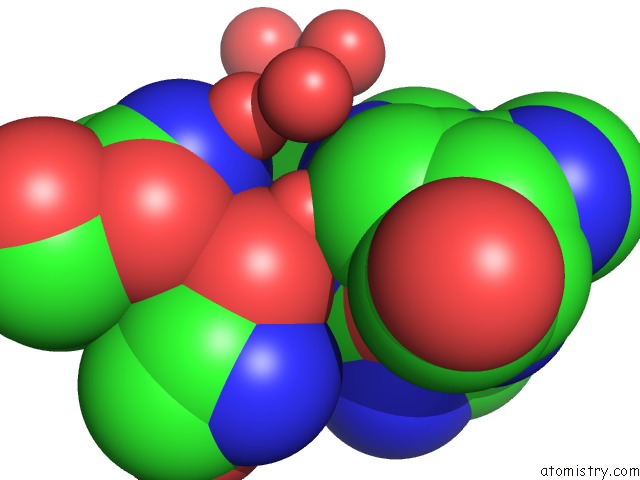
Mono view
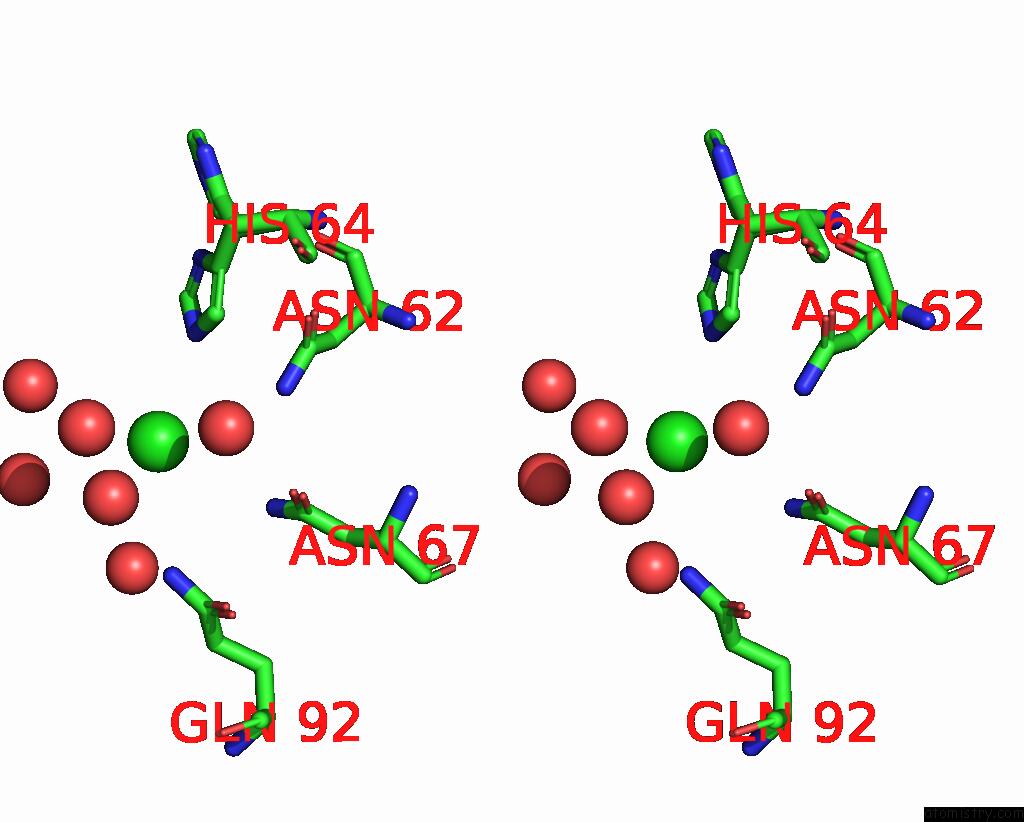
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Effect of Shuttle Location and pH Environment on H+ Transfer in Human Carbonic Anhydrase II within 5.0Å range:
|
Reference:
Z.Fisher,
J.A.Hernandez Prada,
C.K.Tu,
D.Duda,
C.Yoshioka,
H.An,
L.Govindasamy,
D.N.Silverman,
R.Mckenna.
Structural and Kinetic Characterization of Active-Site Histidine As A Proton Shuttle in Catalysis By Human Carbonic Anhydrase II Biochemistry V. 44 1097 2005.
ISSN: ISSN 0006-2960
PubMed: 15667203
DOI: 10.1021/BI0480279
Page generated: Thu Jul 10 19:34:37 2025
ISSN: ISSN 0006-2960
PubMed: 15667203
DOI: 10.1021/BI0480279
Last articles
Cl in 2WODCl in 2WM2
Cl in 2WO4
Cl in 2WNY
Cl in 2WNQ
Cl in 2WMZ
Cl in 2WNT
Cl in 2WNS
Cl in 2WLJ
Cl in 2WML