Chlorine »
PDB 2bdg-2bl9 »
2bj3 »
Chlorine in PDB 2bj3: Nikr-Apo
Protein crystallography data
The structure of Nikr-Apo, PDB code: 2bj3
was solved by
T.H.Tahirov,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.25 / 2.2 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 75.783, 54.322, 77.276, 90.00, 116.06, 90.00 |
| R / Rfree (%) | 21.8 / 26.8 |
Other elements in 2bj3:
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Nikr-Apo
(pdb code 2bj3). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 2 binding sites of Chlorine where determined in the Nikr-Apo, PDB code: 2bj3:
Jump to Chlorine binding site number: 1; 2;
In total 2 binding sites of Chlorine where determined in the Nikr-Apo, PDB code: 2bj3:
Jump to Chlorine binding site number: 1; 2;
Chlorine binding site 1 out of 2 in 2bj3
Go back to
Chlorine binding site 1 out
of 2 in the Nikr-Apo
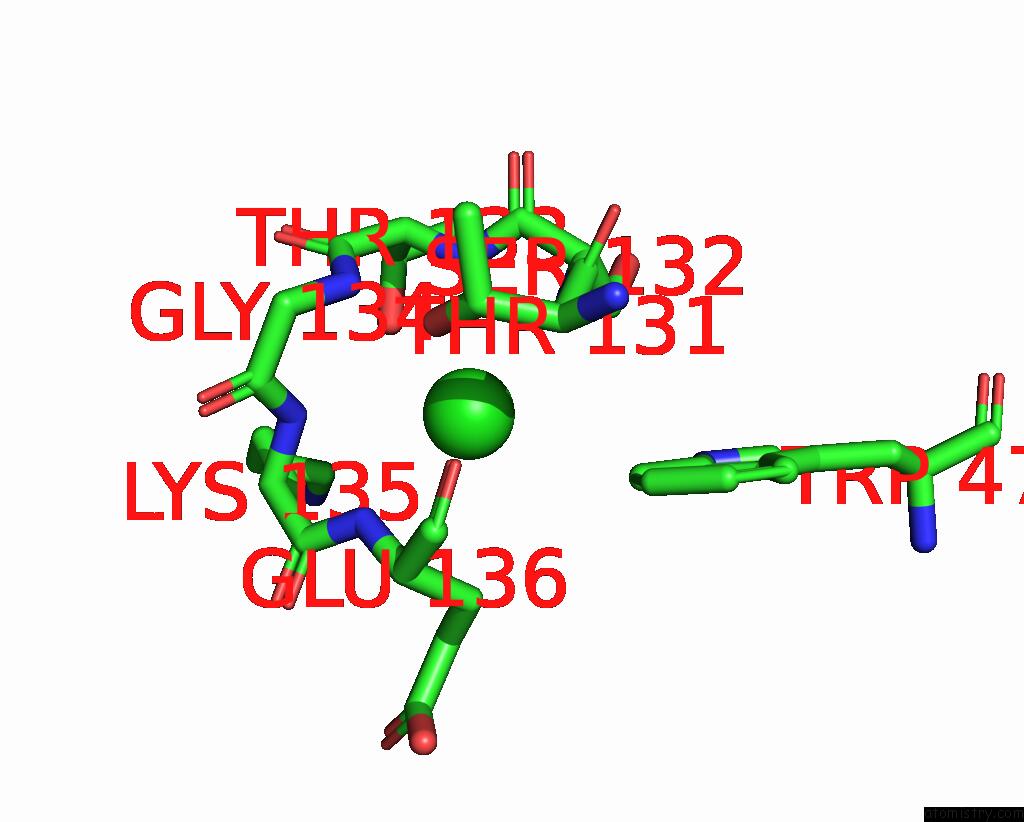
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Nikr-Apo within 5.0Å range:
|
Chlorine binding site 2 out of 2 in 2bj3
Go back to
Chlorine binding site 2 out
of 2 in the Nikr-Apo
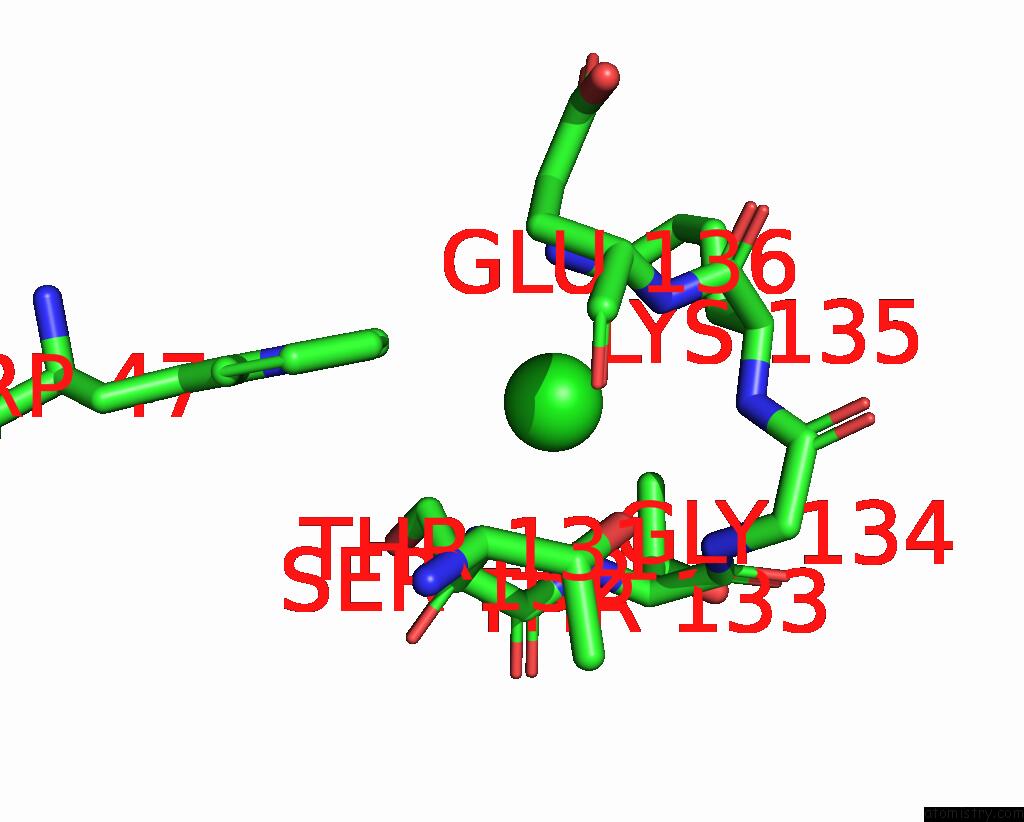
Mono view
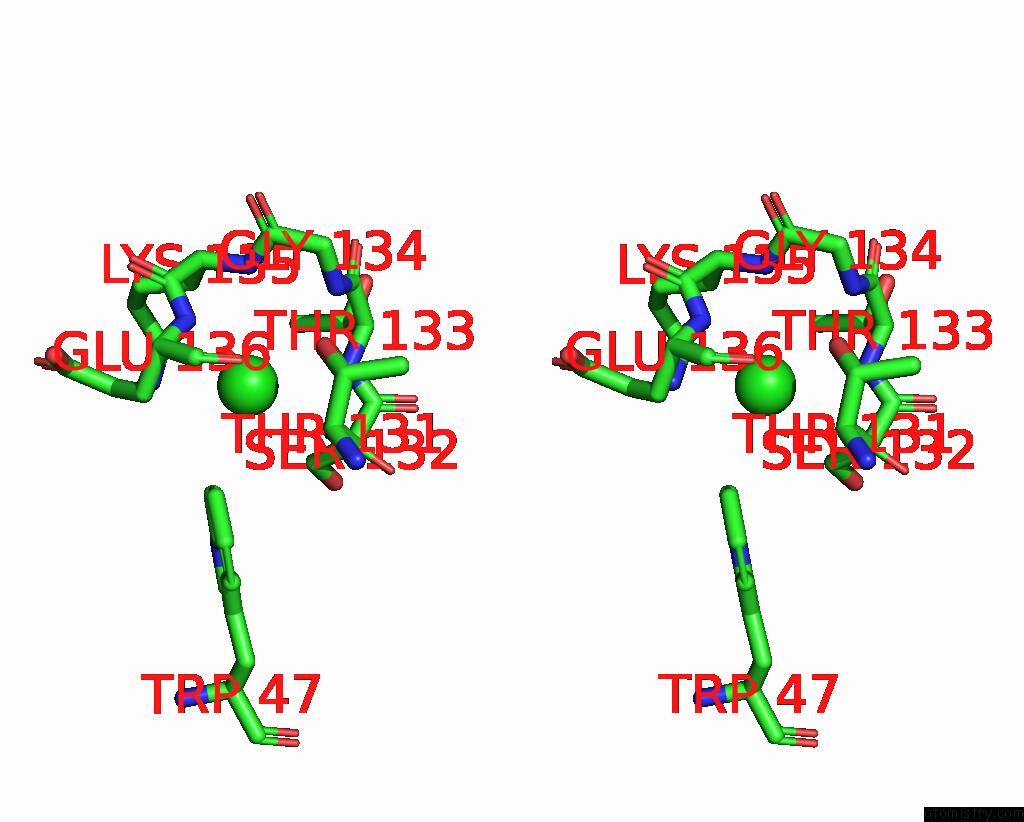
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Nikr-Apo within 5.0Å range:
|
Reference:
P.T.Chivers,
T.H.Tahirov.
Structure of Pyrococcus Horikoshii Nikr: Nickel Sensing and Implications For the Regulation of Dna Recognition J.Mol.Biol. V. 348 597 2005.
ISSN: ISSN 0022-2836
PubMed: 15826657
DOI: 10.1016/J.JMB.2005.03.017
Page generated: Thu Jul 10 21:27:14 2025
ISSN: ISSN 0022-2836
PubMed: 15826657
DOI: 10.1016/J.JMB.2005.03.017
Last articles
F in 7PJCF in 7PJ2
F in 7PHN
F in 7PG6
F in 7PHJ
F in 7PAV
F in 7PH1
F in 7PCD
F in 7P80
F in 7PAW