Chlorine »
PDB 2wjq-2wpr »
2wpr »
Chlorine in PDB 2wpr: Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion)
Protein crystallography data
The structure of Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion), PDB code: 2wpr
was solved by
M.D.Hartmann,
O.Ridderbusch,
A.N.Lupas,
B.Hernandez Alvarez,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 35.36 / 2.65 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 36.130, 62.530, 172.570, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 26.704 / 32.729 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion)
(pdb code 2wpr). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 4 binding sites of Chlorine where determined in the Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion), PDB code: 2wpr:
Jump to Chlorine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Chlorine where determined in the Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion), PDB code: 2wpr:
Jump to Chlorine binding site number: 1; 2; 3; 4;
Chlorine binding site 1 out of 4 in 2wpr
Go back to
Chlorine binding site 1 out
of 4 in the Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion)
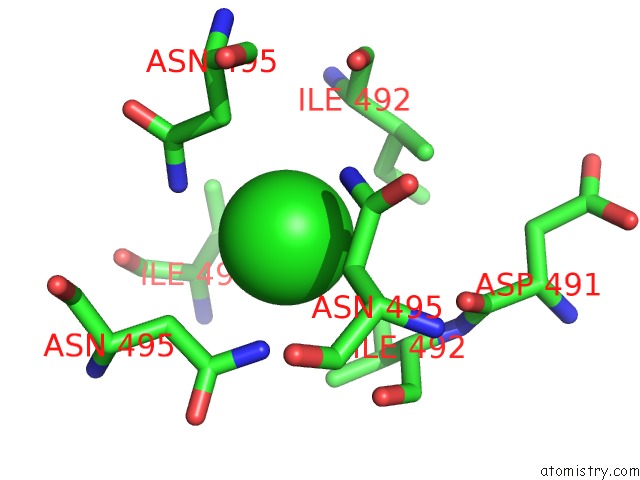
Mono view
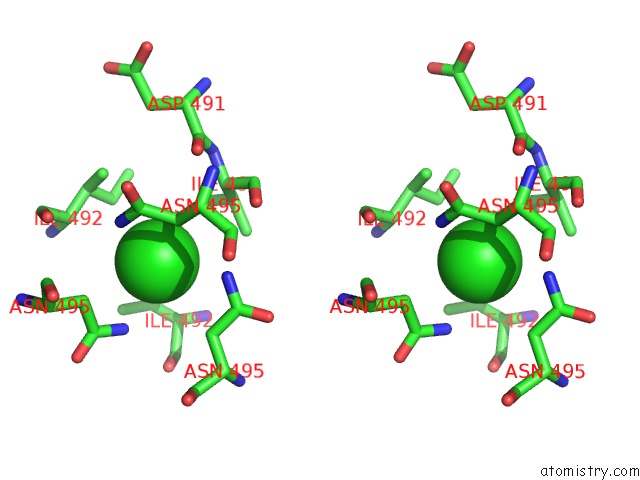
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion) within 5.0Å range:
|
Chlorine binding site 2 out of 4 in 2wpr
Go back to
Chlorine binding site 2 out
of 4 in the Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion)
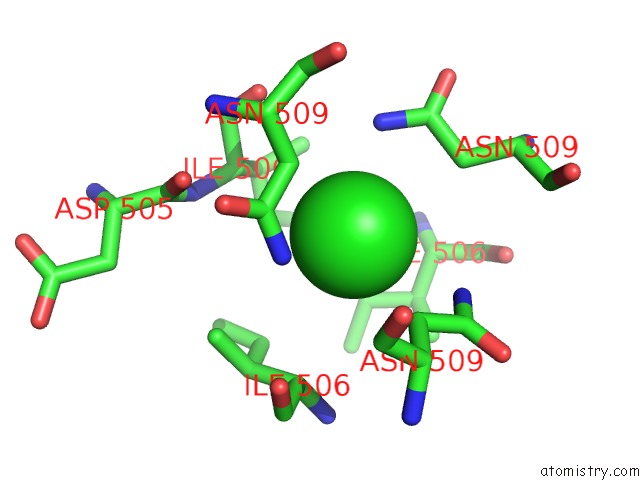
Mono view
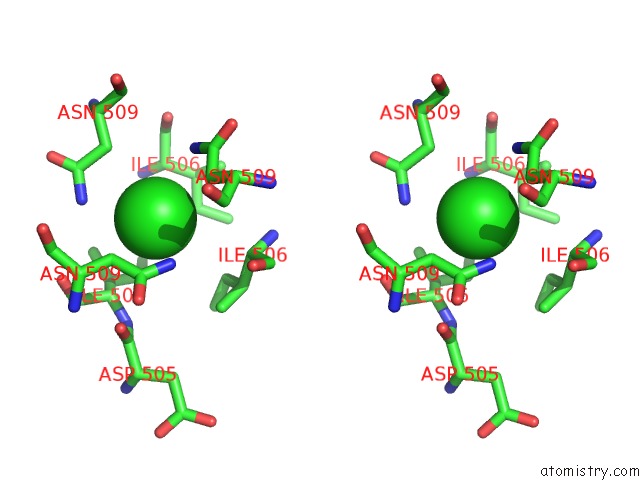
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion) within 5.0Å range:
|
Chlorine binding site 3 out of 4 in 2wpr
Go back to
Chlorine binding site 3 out
of 4 in the Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion)
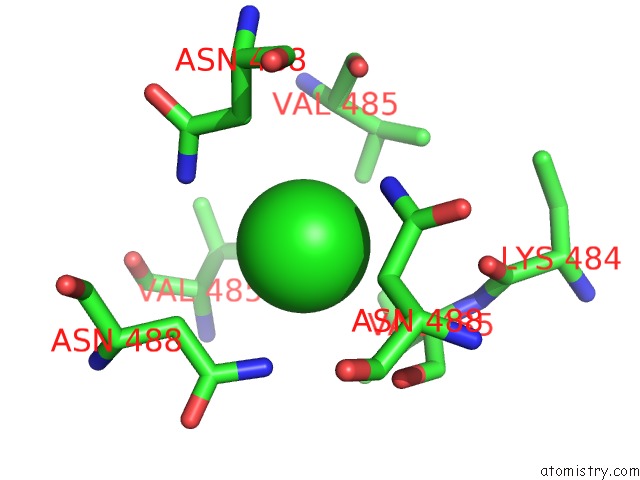
Mono view
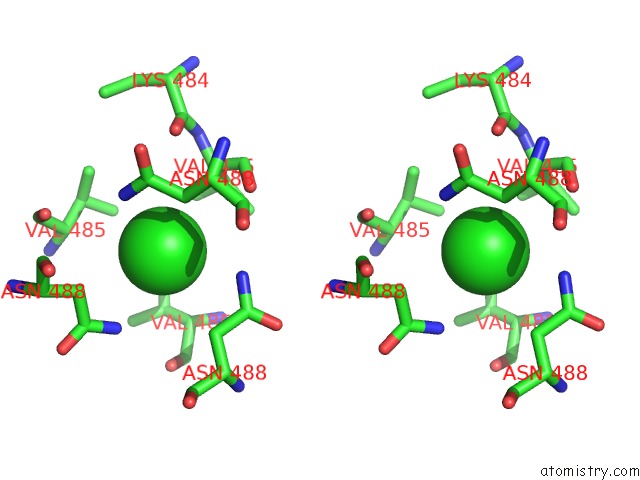
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 3 of Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion) within 5.0Å range:
|
Chlorine binding site 4 out of 4 in 2wpr
Go back to
Chlorine binding site 4 out
of 4 in the Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion)
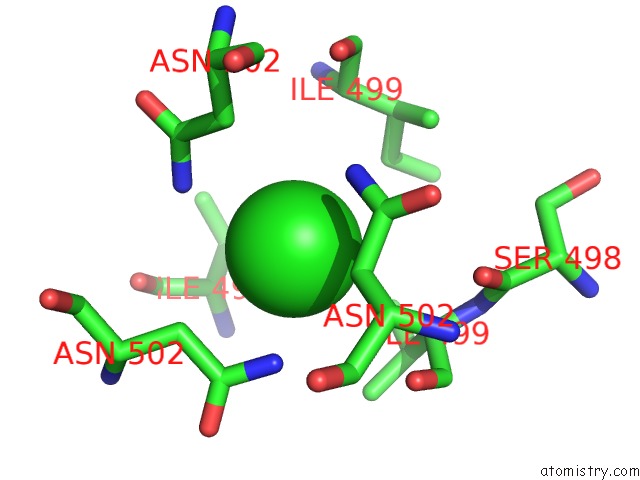
Mono view
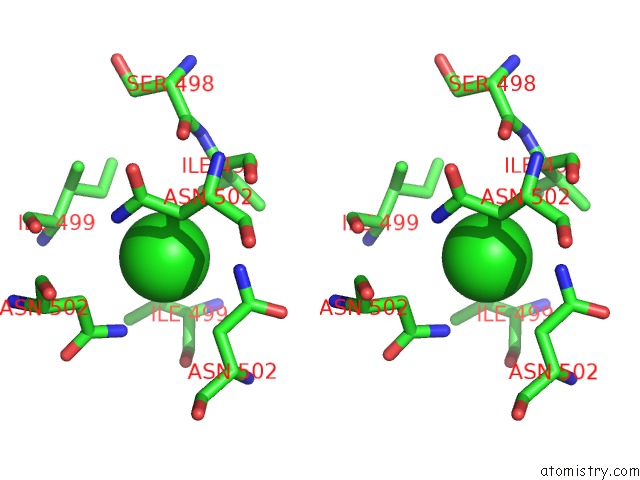
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 4 of Salmonella Enterica Sada 483-523 Fused to GCN4 Adaptors ( SADAK3B-V1, Out-of-Register Fusion) within 5.0Å range:
|
Reference:
M.D.Hartmann,
O.Ridderbusch,
K.Zeth,
R.Albrecht,
O.Testa,
D.N.Woolfson,
G.Sauer,
S.Dunin-Horkawicz,
A.N.Lupas,
B.H.Alvarez.
A Coiled-Coil Motif That Sequesters Ions to the Hydrophobic Core. Proc.Natl.Acad.Sci.Usa V. 106 16950 2009.
ISSN: ISSN 0027-8424
PubMed: 19805097
DOI: 10.1073/PNAS.0907256106
Page generated: Fri Jul 11 01:35:06 2025
ISSN: ISSN 0027-8424
PubMed: 19805097
DOI: 10.1073/PNAS.0907256106
Last articles
F in 7RJ4F in 7RJ2
F in 7RHN
F in 7RME
F in 7RKW
F in 7RKT
F in 7RKR
F in 7RJE
F in 7RJ8
F in 7RH7