Chlorine »
PDB 3g72-3gjd »
3gai »
Chlorine in PDB 3gai: Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp
Enzymatic activity of Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp
All present enzymatic activity of Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp:
2.5.1.17;
2.5.1.17;
Protein crystallography data
The structure of Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp, PDB code: 3gai
was solved by
M.St Maurice,
P.E.Mera,
J.C.Escalante-Semerena,
I.Rayment,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.00 / 1.48 |
| Space group | H 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 80.745, 80.745, 89.693, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 15.6 / 17.4 |
Other elements in 3gai:
The structure of Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp also contains other interesting chemical elements:
| Cobalt | (Co) | 1 atom |
| Magnesium | (Mg) | 1 atom |
| Potassium | (K) | 2 atoms |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp
(pdb code 3gai). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 2 binding sites of Chlorine where determined in the Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp, PDB code: 3gai:
Jump to Chlorine binding site number: 1; 2;
In total 2 binding sites of Chlorine where determined in the Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp, PDB code: 3gai:
Jump to Chlorine binding site number: 1; 2;
Chlorine binding site 1 out of 2 in 3gai
Go back to
Chlorine binding site 1 out
of 2 in the Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp
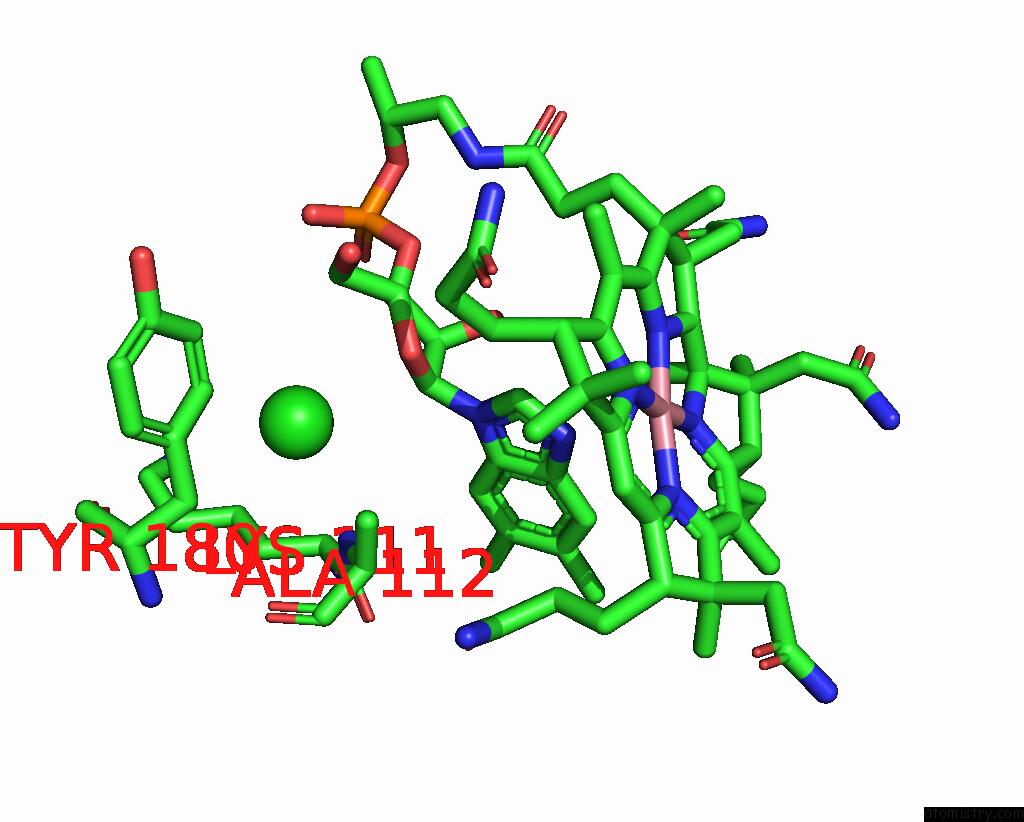
Mono view
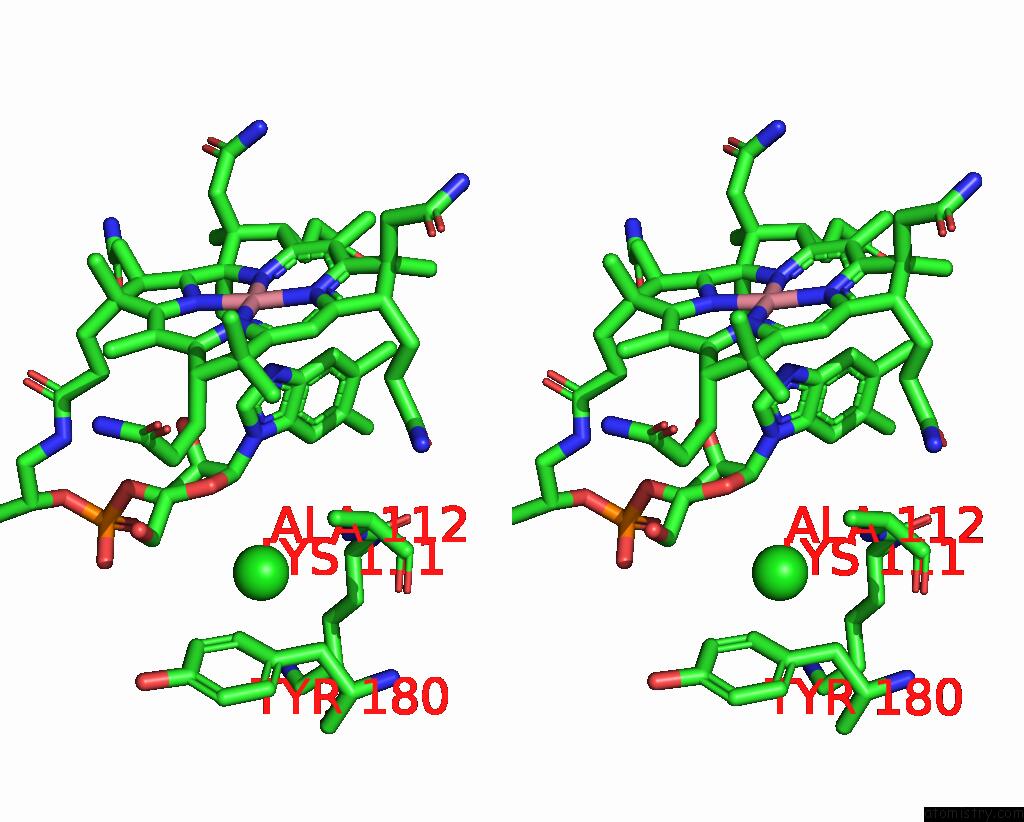
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp within 5.0Å range:
|
Chlorine binding site 2 out of 2 in 3gai
Go back to
Chlorine binding site 2 out
of 2 in the Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp
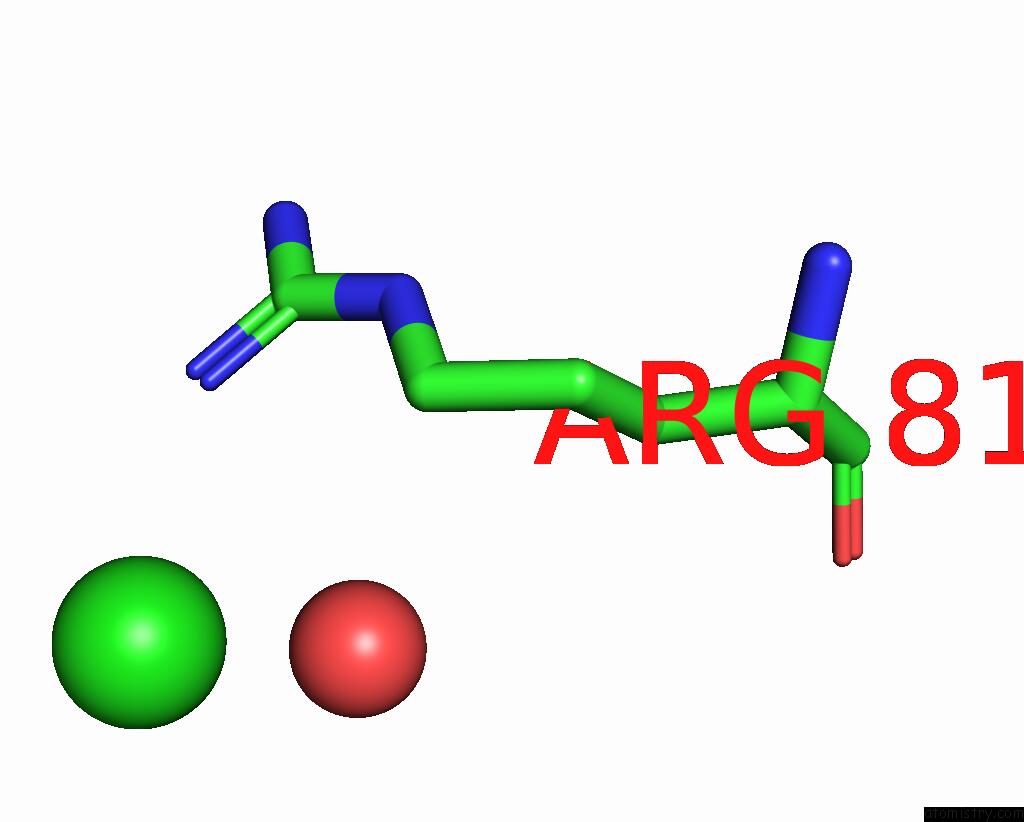
Mono view
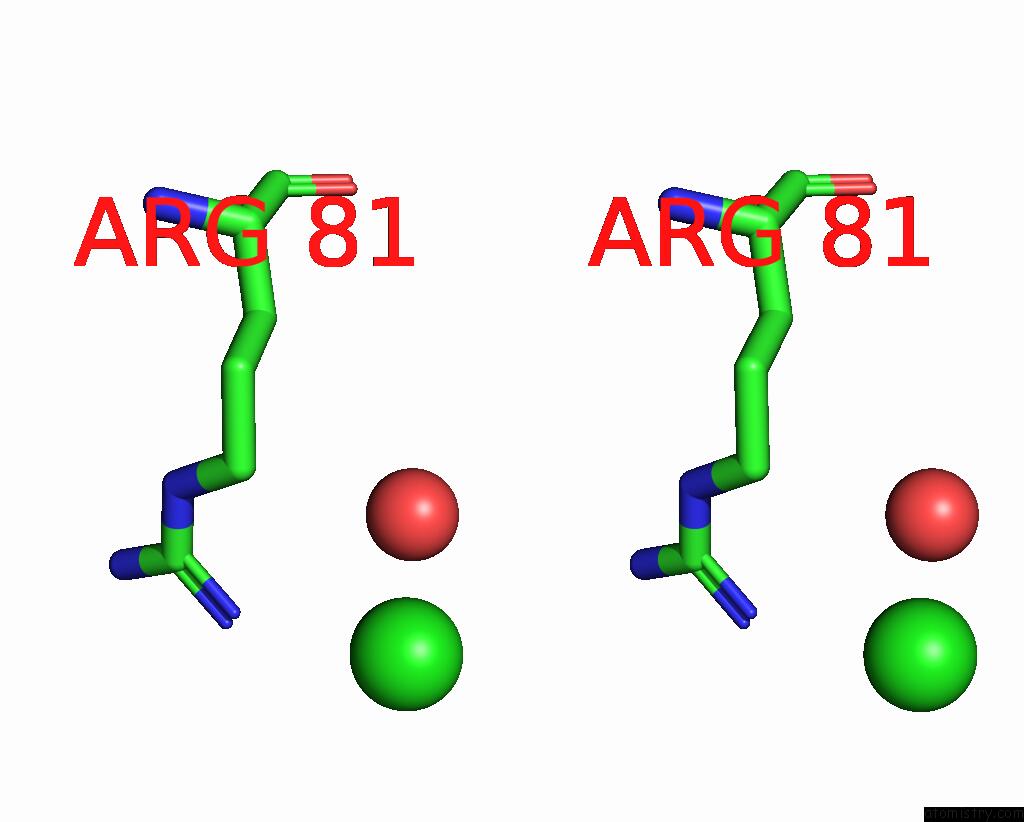
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Structure of A F112A Variant Pduo-Type Atp:Corrinoid Adenosyltransferase From Lactobacillus Reuteri Complexed with Cobalamin and Atp within 5.0Å range:
|
Reference:
P.E.Mera,
M.St Maurice,
I.Rayment,
J.C.Escalante-Semerena.
Residue PHE112 of the Human-Type Corrinoid Adenosyltransferase (Pduo) Enzyme of Lactobacillus Reuteri Is Critical to the Formation of the Four-Coordinate Co(II) Corrinoid Substrate and to the Activity of the Enzyme. Biochemistry V. 48 3138 2009.
ISSN: ISSN 0006-2960
PubMed: 19236001
DOI: 10.1021/BI9000134
Page generated: Sat Jul 20 20:12:03 2024
ISSN: ISSN 0006-2960
PubMed: 19236001
DOI: 10.1021/BI9000134
Last articles
Zn in 9JPJZn in 9JP7
Zn in 9JPK
Zn in 9JPL
Zn in 9GN6
Zn in 9GN7
Zn in 9GKU
Zn in 9GKW
Zn in 9GKX
Zn in 9GL0