Chlorine »
PDB 3ku9-3l29 »
3kw0 »
Chlorine in PDB 3kw0: Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
Protein crystallography data
The structure of Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution, PDB code: 3kw0
was solved by
Joint Center For Structural Genomics (Jcsg),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 37.77 / 2.50 |
| Space group | P 65 |
| Cell size a, b, c (Å), α, β, γ (°) | 64.960, 64.960, 407.800, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 19.2 / 21.9 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
(pdb code 3kw0). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 9 binding sites of Chlorine where determined in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution, PDB code: 3kw0:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9;
In total 9 binding sites of Chlorine where determined in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution, PDB code: 3kw0:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9;
Chlorine binding site 1 out of 9 in 3kw0
Go back to
Chlorine binding site 1 out
of 9 in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
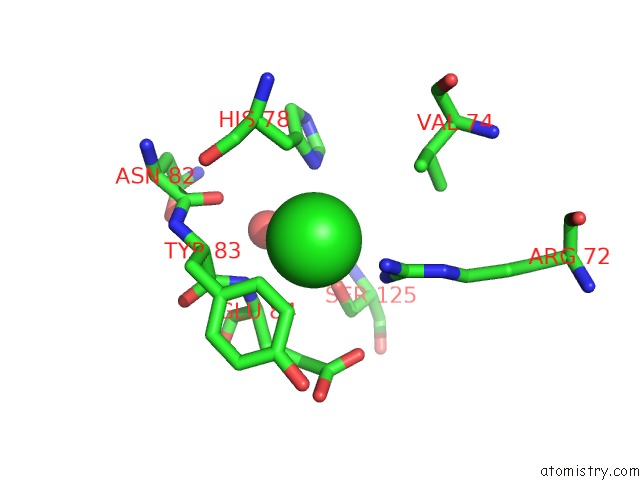
Mono view
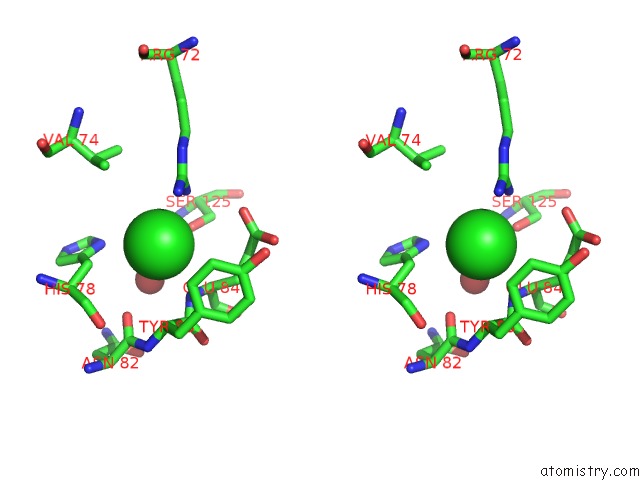
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution within 5.0Å range:
|
Chlorine binding site 2 out of 9 in 3kw0
Go back to
Chlorine binding site 2 out
of 9 in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
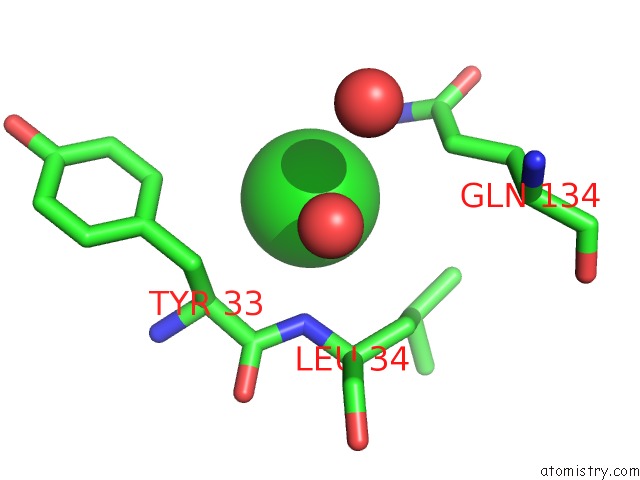
Mono view
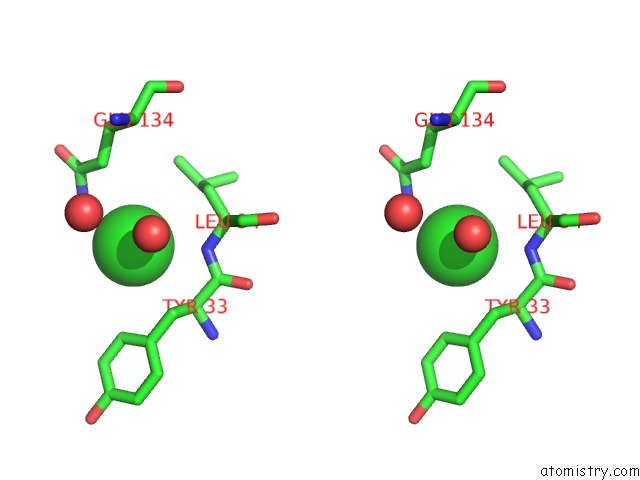
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution within 5.0Å range:
|
Chlorine binding site 3 out of 9 in 3kw0
Go back to
Chlorine binding site 3 out
of 9 in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
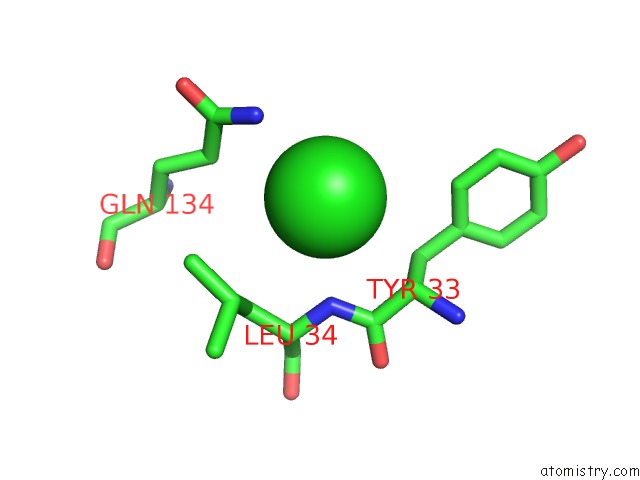
Mono view
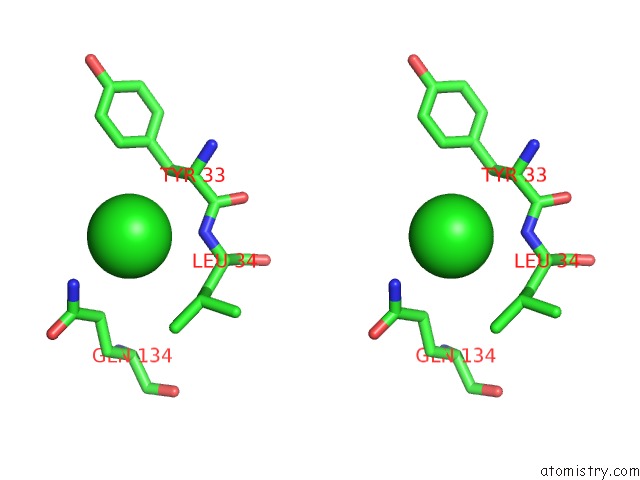
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 3 of Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution within 5.0Å range:
|
Chlorine binding site 4 out of 9 in 3kw0
Go back to
Chlorine binding site 4 out
of 9 in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
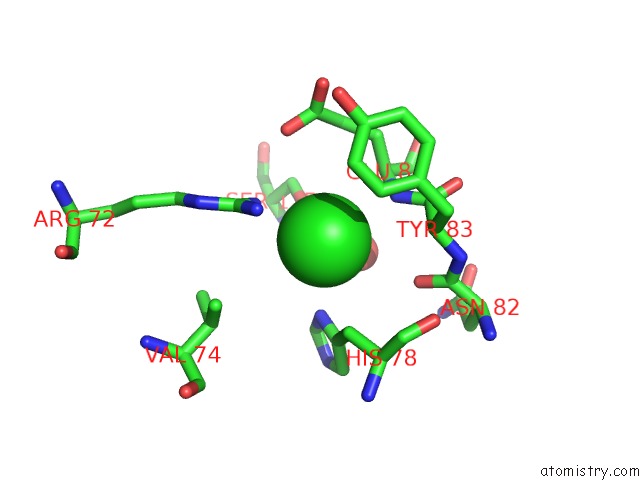
Mono view
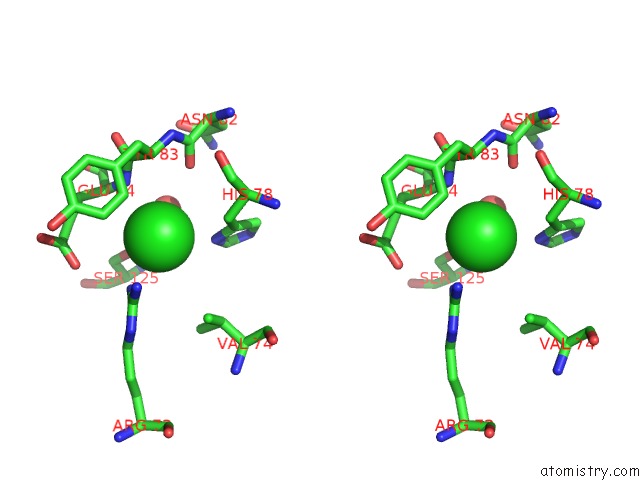
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 4 of Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution within 5.0Å range:
|
Chlorine binding site 5 out of 9 in 3kw0
Go back to
Chlorine binding site 5 out
of 9 in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
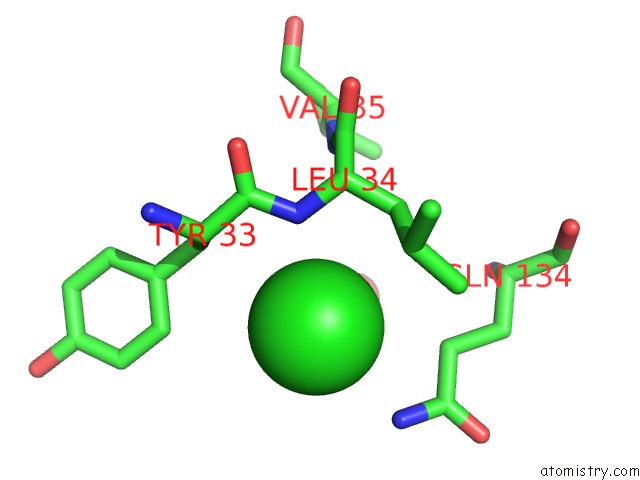
Mono view
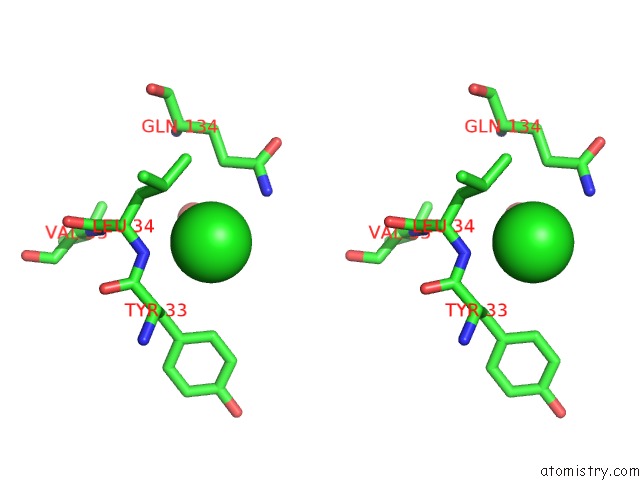
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 5 of Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution within 5.0Å range:
|
Chlorine binding site 6 out of 9 in 3kw0
Go back to
Chlorine binding site 6 out
of 9 in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
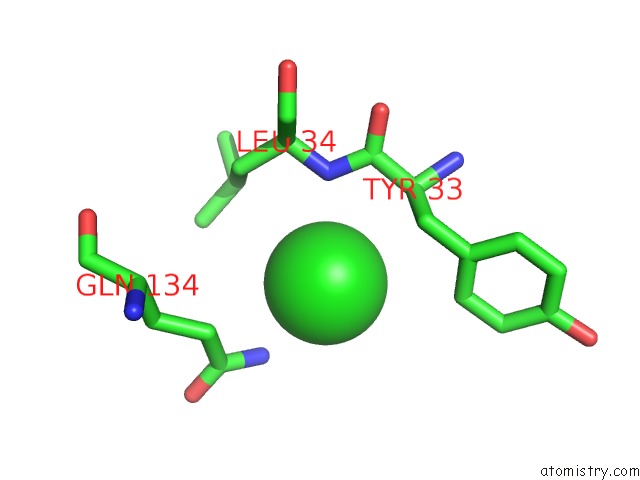
Mono view
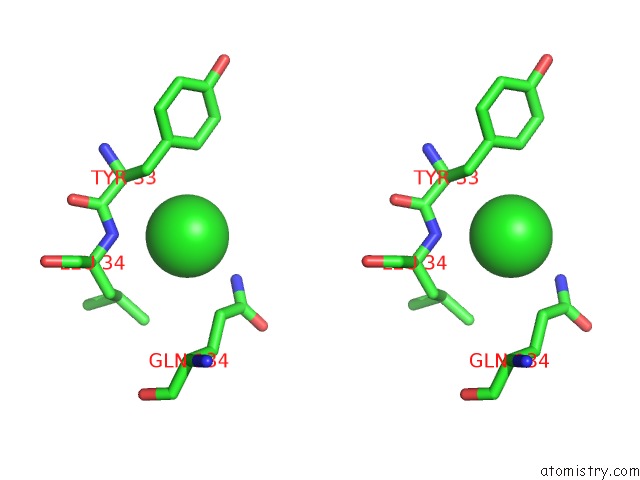
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 6 of Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution within 5.0Å range:
|
Chlorine binding site 7 out of 9 in 3kw0
Go back to
Chlorine binding site 7 out
of 9 in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
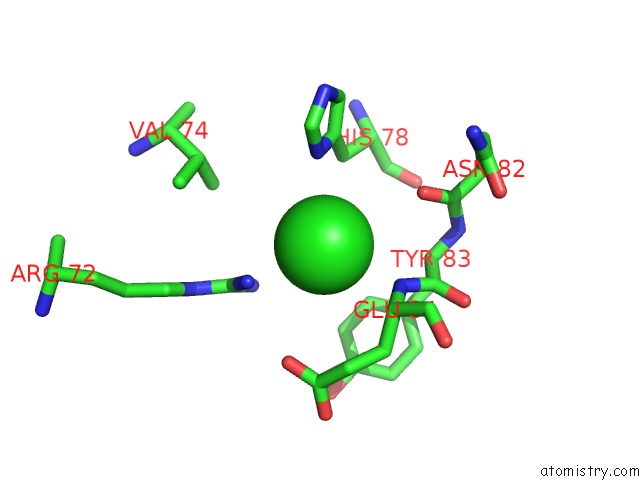
Mono view
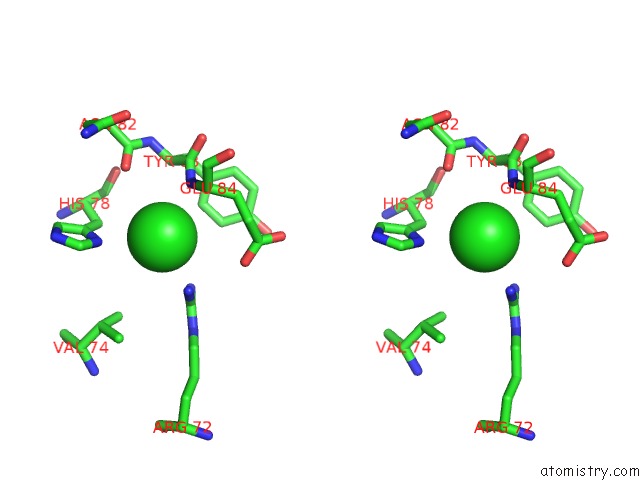
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 7 of Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution within 5.0Å range:
|
Chlorine binding site 8 out of 9 in 3kw0
Go back to
Chlorine binding site 8 out
of 9 in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
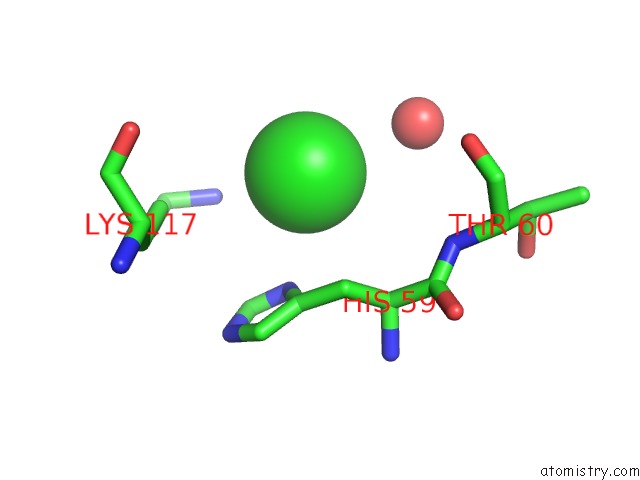
Mono view
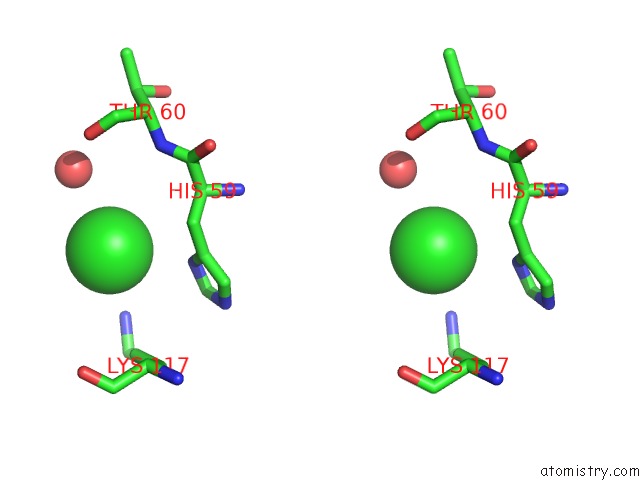
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 8 of Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution within 5.0Å range:
|
Chlorine binding site 9 out of 9 in 3kw0
Go back to
Chlorine binding site 9 out
of 9 in the Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution
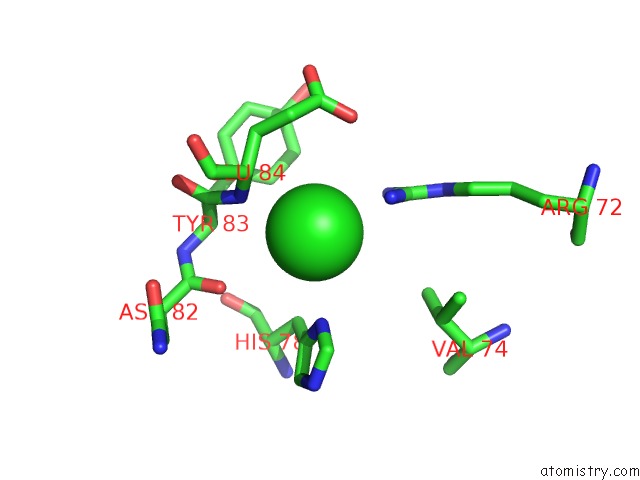
Mono view
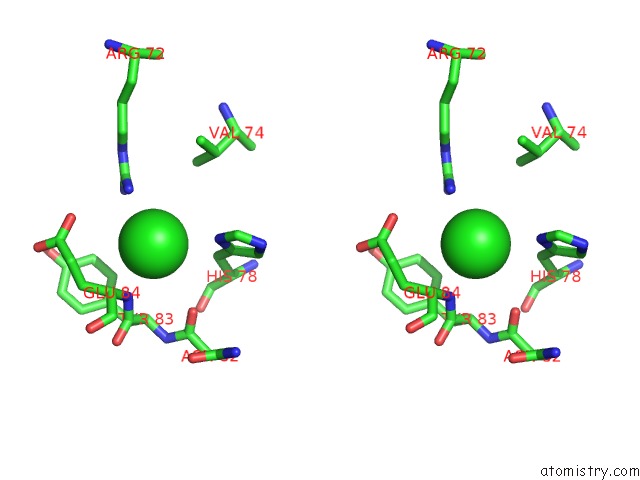
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 9 of Crystal Structure of Cysteine Peptidase (NP_982244.1) From Bacillus Cereus Atcc 10987 at 2.50 A Resolution within 5.0Å range:
|
Reference:
Q.Xu,
N.D.Rawlings,
H.J.Chiu,
L.Jaroszewski,
H.E.Klock,
M.W.Knuth,
M.D.Miller,
M.A.Elsliger,
A.M.Deacon,
A.Godzik,
S.A.Lesley,
I.A.Wilson.
Structural Analysis of Papain-Like Nlpc/P60 Superfamily Enzymes with A Circularly Permuted Topology Reveals Potential Lipid Binding Sites. Plos One V. 6 22013 2011.
ISSN: ESSN 1932-6203
PubMed: 21799766
DOI: 10.1371/JOURNAL.PONE.0022013
Page generated: Fri Jul 11 07:06:31 2025
ISSN: ESSN 1932-6203
PubMed: 21799766
DOI: 10.1371/JOURNAL.PONE.0022013
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO