Chlorine »
PDB 4brl-4c00 »
4buz »
Chlorine in PDB 4buz: SIR2 Complex Structure Mixture of Ex-527 Inhibitor and Reaction Products or of Reaction Substrates P53 Peptide and Nad
Protein crystallography data
The structure of SIR2 Complex Structure Mixture of Ex-527 Inhibitor and Reaction Products or of Reaction Substrates P53 Peptide and Nad, PDB code: 4buz
was solved by
M.Weyand,
M.Lakshminarasimhan,
M.Gertz,
C.Steegborn,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.32 / 1.90 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 46.750, 59.920, 109.030, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 15.93 / 19.357 |
Other elements in 4buz:
The structure of SIR2 Complex Structure Mixture of Ex-527 Inhibitor and Reaction Products or of Reaction Substrates P53 Peptide and Nad also contains other interesting chemical elements:
| Zinc | (Zn) | 1 atom |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the SIR2 Complex Structure Mixture of Ex-527 Inhibitor and Reaction Products or of Reaction Substrates P53 Peptide and Nad
(pdb code 4buz). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the SIR2 Complex Structure Mixture of Ex-527 Inhibitor and Reaction Products or of Reaction Substrates P53 Peptide and Nad, PDB code: 4buz:
In total only one binding site of Chlorine was determined in the SIR2 Complex Structure Mixture of Ex-527 Inhibitor and Reaction Products or of Reaction Substrates P53 Peptide and Nad, PDB code: 4buz:
Chlorine binding site 1 out of 1 in 4buz
Go back to
Chlorine binding site 1 out
of 1 in the SIR2 Complex Structure Mixture of Ex-527 Inhibitor and Reaction Products or of Reaction Substrates P53 Peptide and Nad
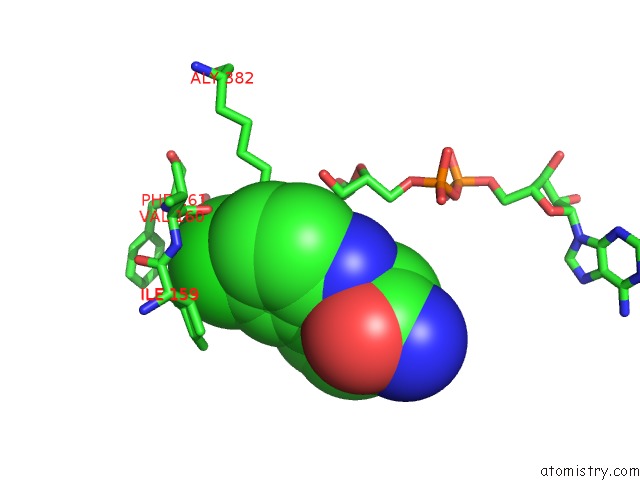
Mono view
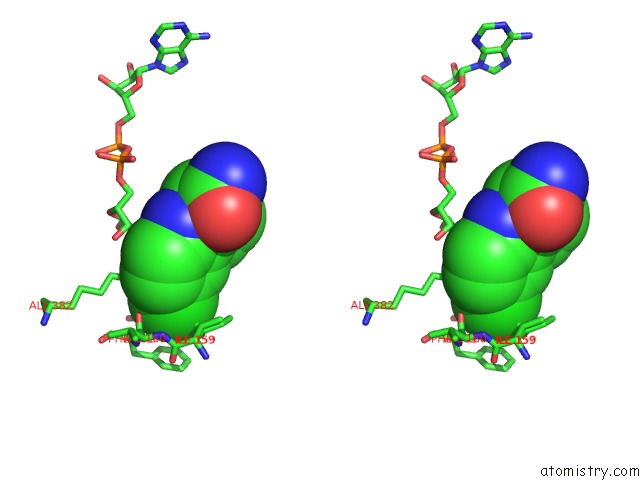
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of SIR2 Complex Structure Mixture of Ex-527 Inhibitor and Reaction Products or of Reaction Substrates P53 Peptide and Nad within 5.0Å range:
|
Reference:
M.Gertz,
F.Fischer,
G.T.T.Nguyen,
M.Lakshminarasimhan,
M.Schutkowski,
M.Weyand,
C.Steegborn.
Ex-527 Inhibits Sirtuins By Exploiting Their Unique Nad+-Dependent Deacetylation Mechanism Proc.Natl.Acad.Sci.Usa V. 110 E2772 2013.
ISSN: ISSN 0027-8424
PubMed: 23840057
DOI: 10.1073/PNAS.1303628110
Page generated: Sun Jul 21 10:34:22 2024
ISSN: ISSN 0027-8424
PubMed: 23840057
DOI: 10.1073/PNAS.1303628110
Last articles
Ca in 5O2YCa in 5O0Z
Ca in 5O25
Ca in 5O1U
Ca in 5O0S
Ca in 5NZE
Ca in 5NZ4
Ca in 5NWE
Ca in 5NYY
Ca in 5NXL