Chlorine »
PDB 4gm6-4gt9 »
4gpz »
Chlorine in PDB 4gpz: Crystal Structure of Human B Type Phosphoglycerate Mutase H11 Phosphorylated Form
Enzymatic activity of Crystal Structure of Human B Type Phosphoglycerate Mutase H11 Phosphorylated Form
All present enzymatic activity of Crystal Structure of Human B Type Phosphoglycerate Mutase H11 Phosphorylated Form:
3.1.3.13; 5.4.2.1; 5.4.2.4;
3.1.3.13; 5.4.2.1; 5.4.2.4;
Protein crystallography data
The structure of Crystal Structure of Human B Type Phosphoglycerate Mutase H11 Phosphorylated Form, PDB code: 4gpz
was solved by
C.He,
L.Zhou,
L.Zhang,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 60.39 / 1.65 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 77.360, 81.174, 90.381, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.5 / 21.4 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Crystal Structure of Human B Type Phosphoglycerate Mutase H11 Phosphorylated Form
(pdb code 4gpz). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the Crystal Structure of Human B Type Phosphoglycerate Mutase H11 Phosphorylated Form, PDB code: 4gpz:
In total only one binding site of Chlorine was determined in the Crystal Structure of Human B Type Phosphoglycerate Mutase H11 Phosphorylated Form, PDB code: 4gpz:
Chlorine binding site 1 out of 1 in 4gpz
Go back to
Chlorine binding site 1 out
of 1 in the Crystal Structure of Human B Type Phosphoglycerate Mutase H11 Phosphorylated Form
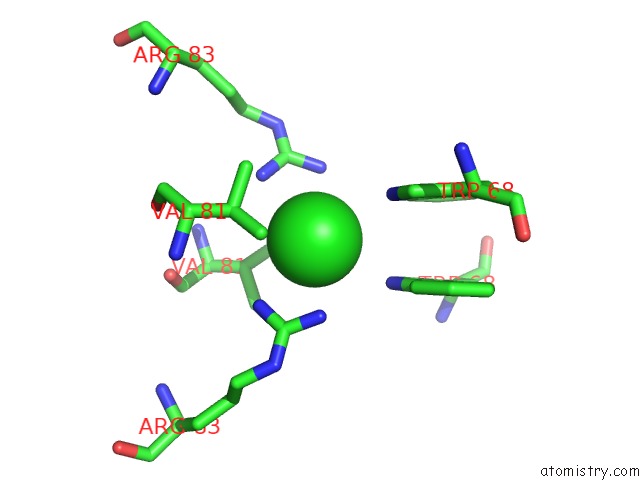
Mono view
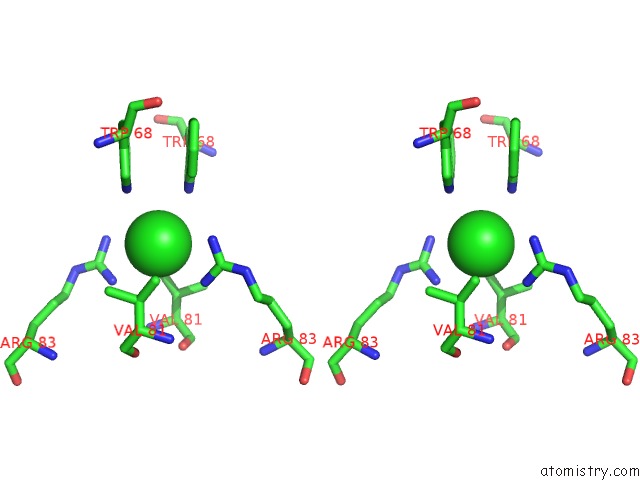
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Crystal Structure of Human B Type Phosphoglycerate Mutase H11 Phosphorylated Form within 5.0Å range:
|
Reference:
T.Hitosugi,
L.Zhou,
J.Fan,
S.Elf,
L.Zhang,
J.Xie,
Y.Wang,
T.L.Gu,
M.Aleckovic,
G.Leroy,
Y.Kang,
H.B.Kang,
J.H.Seo,
C.Shan,
P.Jin,
W.Gong,
S.Lonial,
M.L.Arellano,
H.J.Khoury,
G.Z.Chen,
D.M.Shin,
F.R.Khuri,
T.J.Boggon,
S.Kang,
C.He,
J.Chen.
TYR26 Phosphorylation of PGAM1 Provides A Metabolic Advantage to Tumours By Stabilizing the Active Conformation. Nat Commun V. 4 1790 2013.
ISSN: ESSN 2041-1723
PubMed: 23653202
DOI: 10.1038/NCOMMS2759
Page generated: Fri Jul 11 15:49:20 2025
ISSN: ESSN 2041-1723
PubMed: 23653202
DOI: 10.1038/NCOMMS2759
Last articles
Cl in 4Y8NCl in 4Y8J
Cl in 4Y8H
Cl in 4Y8I
Cl in 4Y8K
Cl in 4Y8G
Cl in 4Y8C
Cl in 4Y89
Cl in 4Y88
Cl in 4Y86