Chlorine »
PDB 5mgg-5mls »
5ml9 »
Chlorine in PDB 5ml9: Cocrystal Structure of Fc Gamma Receptor Iiia Interacting with Affimer F4, A Specific Binding Protein Which Blocks Igg Binding to the Receptor.
Protein crystallography data
The structure of Cocrystal Structure of Fc Gamma Receptor Iiia Interacting with Affimer F4, A Specific Binding Protein Which Blocks Igg Binding to the Receptor., PDB code: 5ml9
was solved by
J.I.Robinson,
D.C.Tomlinson,
E.W.Baxter,
R.L.Owen,
M.Thomsen,
S.J.Win,
J.E.Nettleship,
C.Tiede,
R.J.Foster,
M.P.Waterhouse,
S.A.Harris,
R.J.Owens,
C.W.G.Fishwick,
A.Goldman,
M.J.Mcpherson,
A.W.Morgan,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.74 / 2.35 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 56.481, 72.587, 96.451, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.9 / 27.2 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Cocrystal Structure of Fc Gamma Receptor Iiia Interacting with Affimer F4, A Specific Binding Protein Which Blocks Igg Binding to the Receptor.
(pdb code 5ml9). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the Cocrystal Structure of Fc Gamma Receptor Iiia Interacting with Affimer F4, A Specific Binding Protein Which Blocks Igg Binding to the Receptor., PDB code: 5ml9:
In total only one binding site of Chlorine was determined in the Cocrystal Structure of Fc Gamma Receptor Iiia Interacting with Affimer F4, A Specific Binding Protein Which Blocks Igg Binding to the Receptor., PDB code: 5ml9:
Chlorine binding site 1 out of 1 in 5ml9
Go back to
Chlorine binding site 1 out
of 1 in the Cocrystal Structure of Fc Gamma Receptor Iiia Interacting with Affimer F4, A Specific Binding Protein Which Blocks Igg Binding to the Receptor.
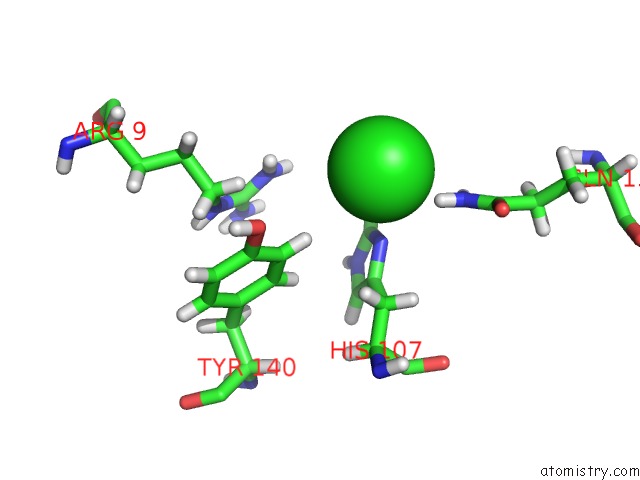
Mono view
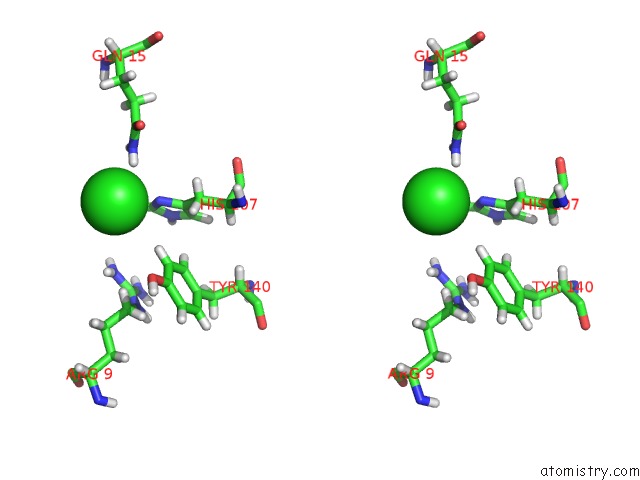
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Cocrystal Structure of Fc Gamma Receptor Iiia Interacting with Affimer F4, A Specific Binding Protein Which Blocks Igg Binding to the Receptor. within 5.0Å range:
|
Reference:
J.I.Robinson,
E.W.Baxter,
R.L.Owen,
M.Thomsen,
D.C.Tomlinson,
M.P.Waterhouse,
S.J.Win,
J.E.Nettleship,
C.Tiede,
R.J.Foster,
R.J.Owens,
C.W.G.Fishwick,
S.A.Harris,
A.Goldman,
M.J.Mcpherson,
A.W.Morgan.
Affimer Proteins Inhibit Immune Complex Binding to Fc Gamma Riiia with High Specificity Through Competitive and Allosteric Modes of Action. Proc. Natl. Acad. Sci. V. 115 E72 2018U.S.A..
ISSN: ESSN 1091-6490
PubMed: 29247053
DOI: 10.1073/PNAS.1707856115
Page generated: Sat Jul 12 05:33:07 2025
ISSN: ESSN 1091-6490
PubMed: 29247053
DOI: 10.1073/PNAS.1707856115
Last articles
F in 4FMDF in 4FME
F in 4FMC
F in 4FMB
F in 4FM5
F in 4FFW
F in 4FM8
F in 4FM7
F in 4FLH
F in 4FIA