Chlorine »
PDB 6hkq-6hs3 »
6hrr »
Chlorine in PDB 6hrr: Structure of the TRPML2 Eld at pH 6.5
Protein crystallography data
The structure of Structure of the TRPML2 Eld at pH 6.5, PDB code: 6hrr
was solved by
N.Bader,
K.K.Viet,
A.Wagner,
U.A.Hellmich,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.00 |
| Space group | I 4 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 109.471, 109.471, 149.744, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.6 / 21.7 |
Other elements in 6hrr:
The structure of Structure of the TRPML2 Eld at pH 6.5 also contains other interesting chemical elements:
| Potassium | (K) | 1 atom |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Structure of the TRPML2 Eld at pH 6.5
(pdb code 6hrr). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 3 binding sites of Chlorine where determined in the Structure of the TRPML2 Eld at pH 6.5, PDB code: 6hrr:
Jump to Chlorine binding site number: 1; 2; 3;
In total 3 binding sites of Chlorine where determined in the Structure of the TRPML2 Eld at pH 6.5, PDB code: 6hrr:
Jump to Chlorine binding site number: 1; 2; 3;
Chlorine binding site 1 out of 3 in 6hrr
Go back to
Chlorine binding site 1 out
of 3 in the Structure of the TRPML2 Eld at pH 6.5

Mono view
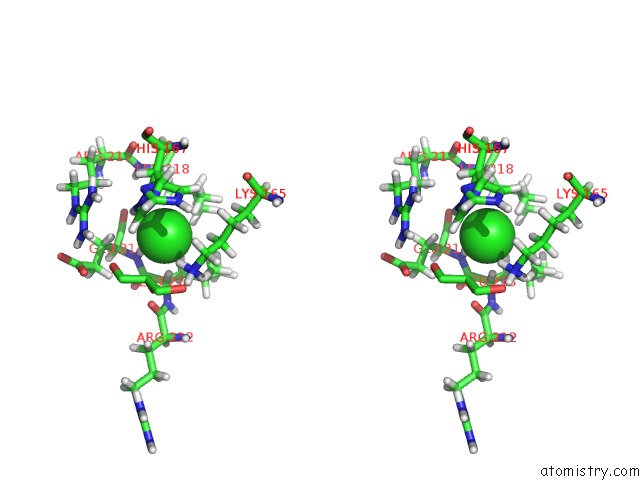
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Structure of the TRPML2 Eld at pH 6.5 within 5.0Å range:
|
Chlorine binding site 2 out of 3 in 6hrr
Go back to
Chlorine binding site 2 out
of 3 in the Structure of the TRPML2 Eld at pH 6.5

Mono view
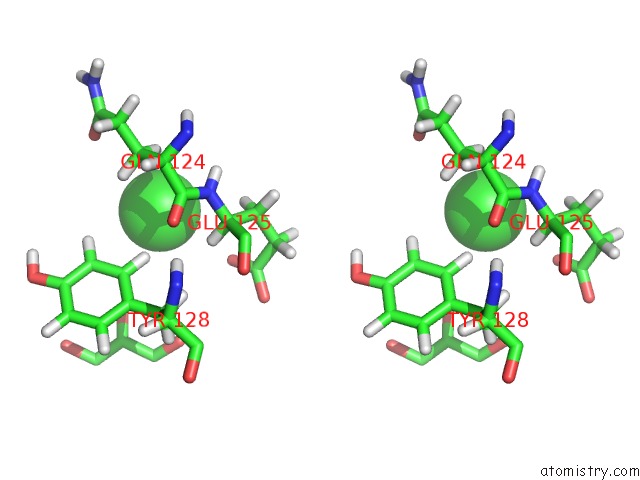
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Structure of the TRPML2 Eld at pH 6.5 within 5.0Å range:
|
Chlorine binding site 3 out of 3 in 6hrr
Go back to
Chlorine binding site 3 out
of 3 in the Structure of the TRPML2 Eld at pH 6.5
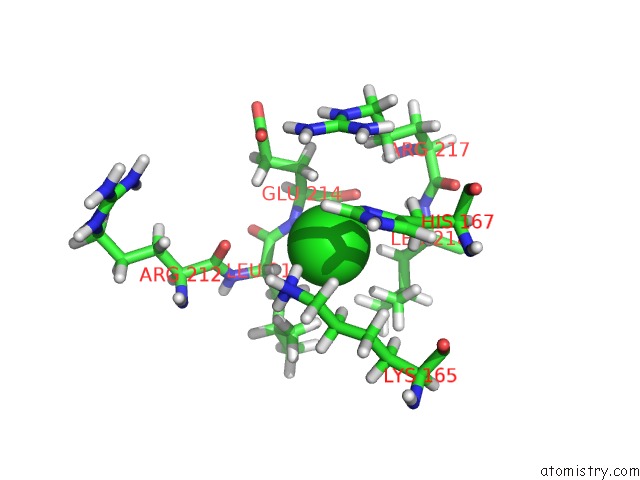
Mono view
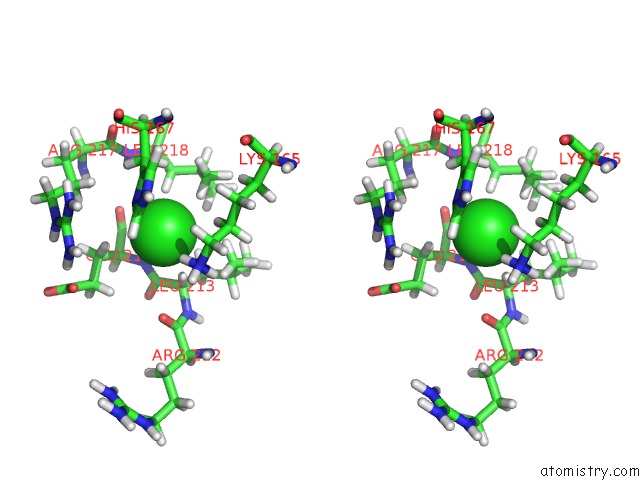
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 3 of Structure of the TRPML2 Eld at pH 6.5 within 5.0Å range:
|
Reference:
K.K.Viet,
A.Wagner,
K.Schwickert,
N.Hellwig,
M.Brennich,
N.Bader,
T.Schirmeister,
N.Morgner,
H.Schindelin,
U.A.Hellmich.
Structure of the Human TRPML2 Ion Channel Extracytosolic/Lumenal Domain. Structure V. 27 1246 2019.
ISSN: ISSN 0969-2126
PubMed: 31178222
DOI: 10.1016/J.STR.2019.04.016
Page generated: Sun Jul 28 01:04:43 2024
ISSN: ISSN 0969-2126
PubMed: 31178222
DOI: 10.1016/J.STR.2019.04.016
Last articles
Cl in 5TWSCl in 5TXU
Cl in 5TXI
Cl in 5TWR
Cl in 5TWO
Cl in 5TWQ
Cl in 5TWL
Cl in 5TW4
Cl in 5TVL
Cl in 5TVT