Chlorine »
PDB 6siq-6sr2 »
6sor »
Chlorine in PDB 6sor: 20 Minute FE2+ Soaked Structure of Synftn Variant E62A
Enzymatic activity of 20 Minute FE2+ Soaked Structure of Synftn Variant E62A
All present enzymatic activity of 20 Minute FE2+ Soaked Structure of Synftn Variant E62A:
1.16.3.2;
1.16.3.2;
Protein crystallography data
The structure of 20 Minute FE2+ Soaked Structure of Synftn Variant E62A, PDB code: 6sor
was solved by
A.M.Hemmings,
J.M.Bradley,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.48 / 1.74 |
| Space group | F 4 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 176.450, 176.450, 176.450, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 15.7 / 18.8 |
Other elements in 6sor:
The structure of 20 Minute FE2+ Soaked Structure of Synftn Variant E62A also contains other interesting chemical elements:
| Iron | (Fe) | 3 atoms |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the 20 Minute FE2+ Soaked Structure of Synftn Variant E62A
(pdb code 6sor). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the 20 Minute FE2+ Soaked Structure of Synftn Variant E62A, PDB code: 6sor:
In total only one binding site of Chlorine was determined in the 20 Minute FE2+ Soaked Structure of Synftn Variant E62A, PDB code: 6sor:
Chlorine binding site 1 out of 1 in 6sor
Go back to
Chlorine binding site 1 out
of 1 in the 20 Minute FE2+ Soaked Structure of Synftn Variant E62A
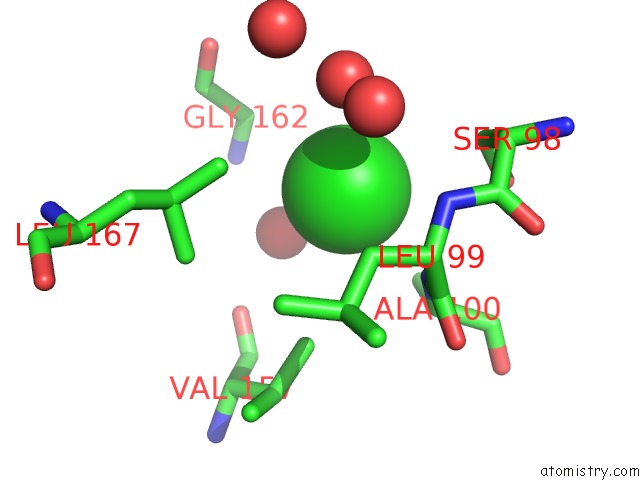
Mono view
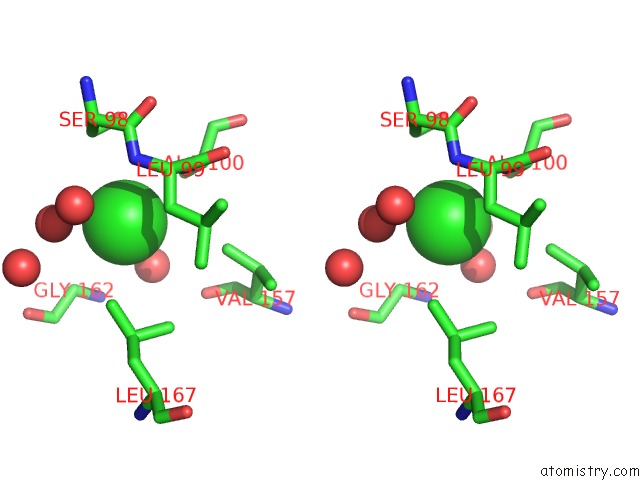
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of 20 Minute FE2+ Soaked Structure of Synftn Variant E62A within 5.0Å range:
|
Reference:
J.M.Bradley,
J.Pullin,
G.R.Moore,
D.A.Svistunenko,
A.M.Hemmings,
N.E.Le Brun.
Routes of Iron Entry Into, and Exit From, the Catalytic Ferroxidase Sites of the Prokaryotic Ferritin Synftn. Dalton Trans 2020.
ISSN: ESSN 1477-9234
PubMed: 31930254
DOI: 10.1039/C9DT03570B
Page generated: Mon Jul 29 15:04:37 2024
ISSN: ESSN 1477-9234
PubMed: 31930254
DOI: 10.1039/C9DT03570B
Last articles
Ca in 2W94Ca in 2W87
Ca in 2W86
Ca in 2W7P
Ca in 2W7O
Ca in 2W67
Ca in 2W68
Ca in 2W4Z
Ca in 2W4Y
Ca in 2W66