Chlorine »
PDB 7ltb-7m0h »
7ly8 »
Chlorine in PDB 7ly8: The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
Enzymatic activity of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
All present enzymatic activity of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring:
4.2.1.20;
4.2.1.20;
Protein crystallography data
The structure of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring, PDB code: 7ly8
was solved by
E.Hilario,
M.F.Dunn,
L.J.Mueller,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.85 / 1.55 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 183.645, 58.8, 67.205, 90, 95.13, 90 |
| R / Rfree (%) | 17.3 / 19.4 |
Other elements in 7ly8:
The structure of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring also contains other interesting chemical elements:
| Fluorine | (F) | 9 atoms |
| Sodium | (Na) | 1 atom |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
(pdb code 7ly8). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 5 binding sites of Chlorine where determined in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring, PDB code: 7ly8:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Chlorine where determined in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring, PDB code: 7ly8:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5;
Chlorine binding site 1 out of 5 in 7ly8
Go back to
Chlorine binding site 1 out
of 5 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
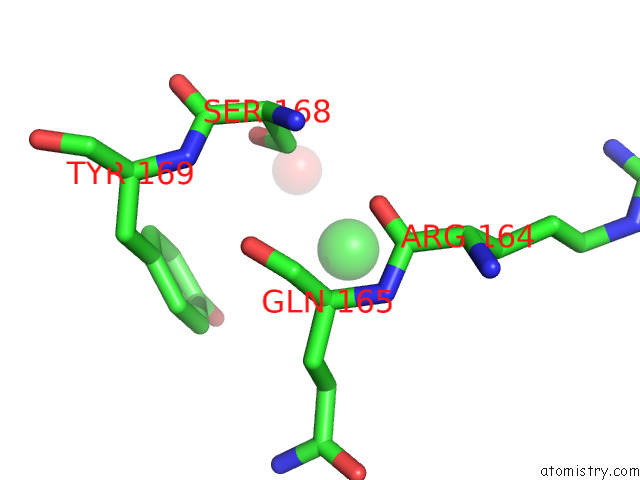
Mono view
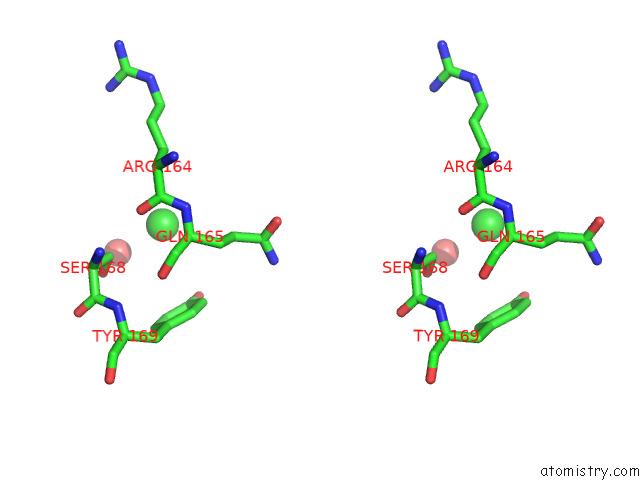
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Chlorine binding site 2 out of 5 in 7ly8
Go back to
Chlorine binding site 2 out
of 5 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
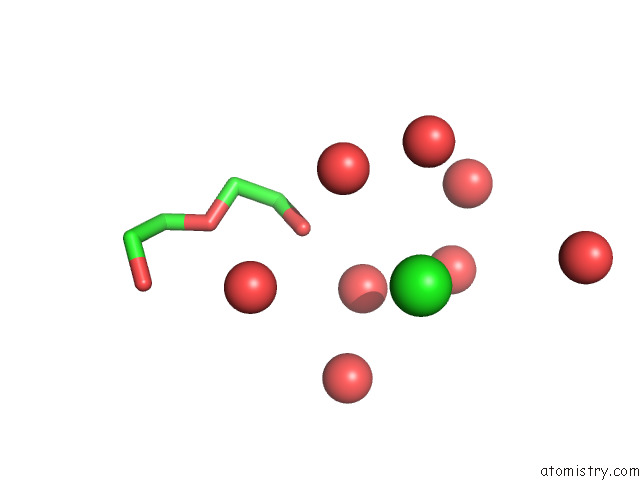
Mono view
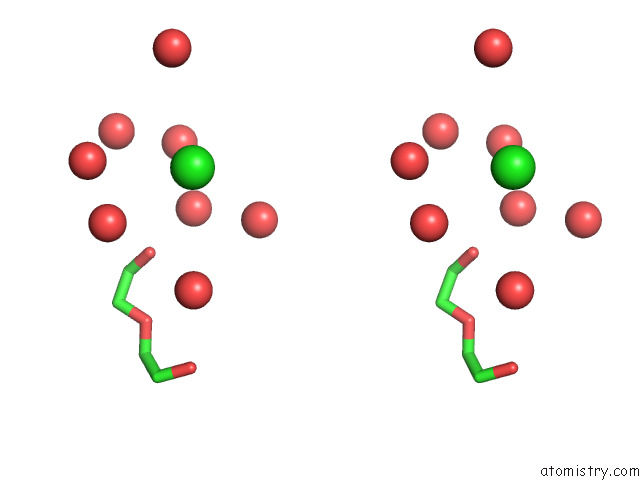
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Chlorine binding site 3 out of 5 in 7ly8
Go back to
Chlorine binding site 3 out
of 5 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
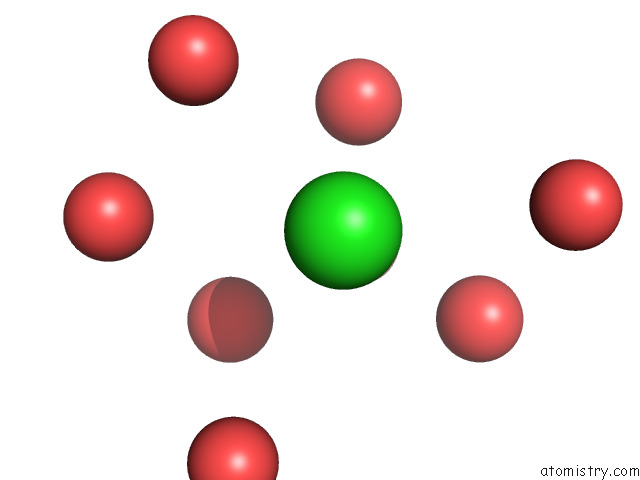
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 3 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Chlorine binding site 4 out of 5 in 7ly8
Go back to
Chlorine binding site 4 out
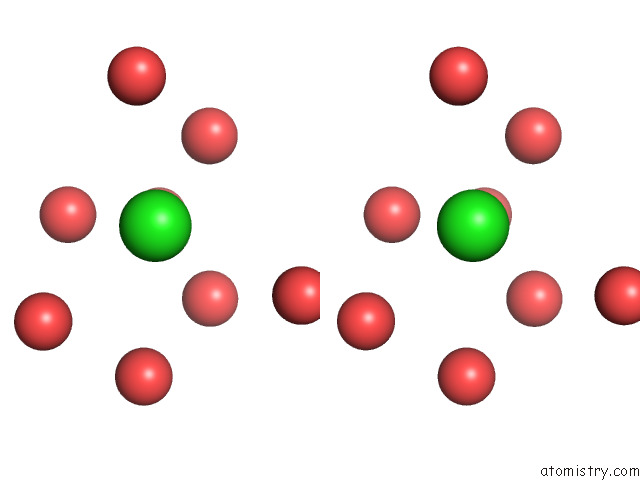
of 5 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 4 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Chlorine binding site 5 out of 5 in 7ly8
Go back to
Chlorine binding site 5 out
of 5 in the The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring
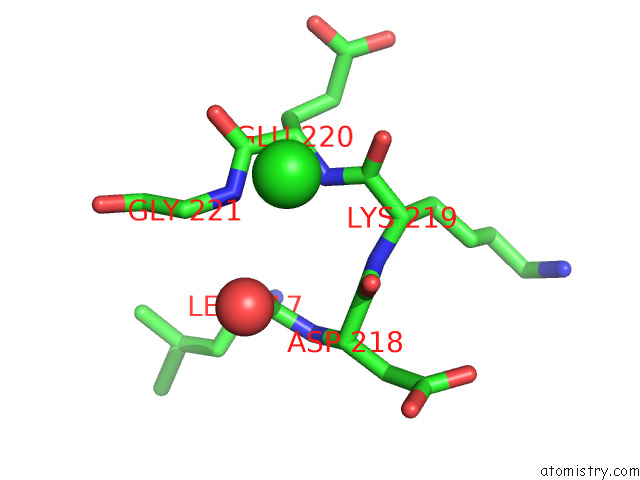
Mono view
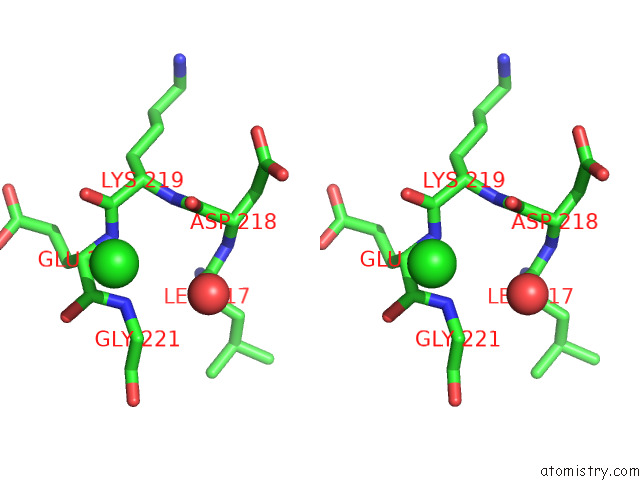
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 5 of The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'- Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring within 5.0Å range:
|
Reference:
E.Hilario,
M.F.Dunn,
L.J.Mueller.
The Internal Aldimine Form of the Wild-Type Salmonella Typhimurium Tryptophan Synthase in Complex with Two Molecules of N-(4'-Trifluoromethoxybenzoyl)-2-Amino-1-Ethylphosphate (F6F) Inhibitor at the Enzyme Alpha-Site, A Single F6F Molecule at the Enzyme Beta-Site, and Sodium Ion at the Metal Coordination Site at 1.55 Angstrom Resolution. One of the Beta-Q114 Rotamer Conformations Allows A Hydrogen Bond to Form with the Plp Oxygen at the Position 3 in the Ring. To Be Published.
Page generated: Sun Jul 13 03:54:48 2025
Last articles
F in 7QQ6F in 7QPZ
F in 7QPY
F in 7QN0
F in 7QN4
F in 7QN1
F in 7QN2
F in 7QN3
F in 7QMY
F in 7QMX