Chlorine »
PDB 8ab7-8alv »
8aio »
Chlorine in PDB 8aio: Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi)
Enzymatic activity of Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi)
All present enzymatic activity of Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi):
1.12.7.2;
1.12.7.2;
Protein crystallography data
The structure of Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi), PDB code: 8aio
was solved by
J.Duan,
E.Hofmann,
T.Happe,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.03 / 1.52 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 87.6, 72.16, 103.21, 90, 101.94, 90 |
| R / Rfree (%) | 16.7 / 19.2 |
Other elements in 8aio:
The structure of Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi) also contains other interesting chemical elements:
| Magnesium | (Mg) | 4 atoms |
| Iron | (Fe) | 40 atoms |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi)
(pdb code 8aio). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 2 binding sites of Chlorine where determined in the Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi), PDB code: 8aio:
Jump to Chlorine binding site number: 1; 2;
In total 2 binding sites of Chlorine where determined in the Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi), PDB code: 8aio:
Jump to Chlorine binding site number: 1; 2;
Chlorine binding site 1 out of 2 in 8aio
Go back to
Chlorine binding site 1 out
of 2 in the Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi)
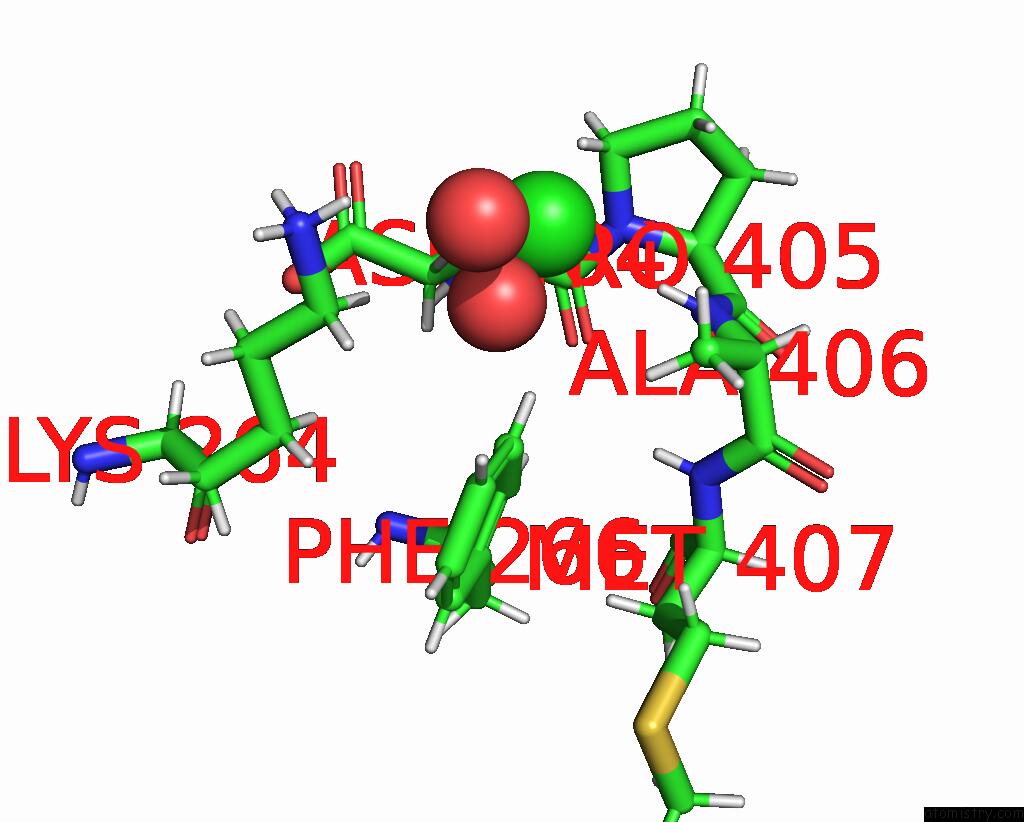
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi) within 5.0Å range:
|
Chlorine binding site 2 out of 2 in 8aio
Go back to
Chlorine binding site 2 out
of 2 in the Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi)
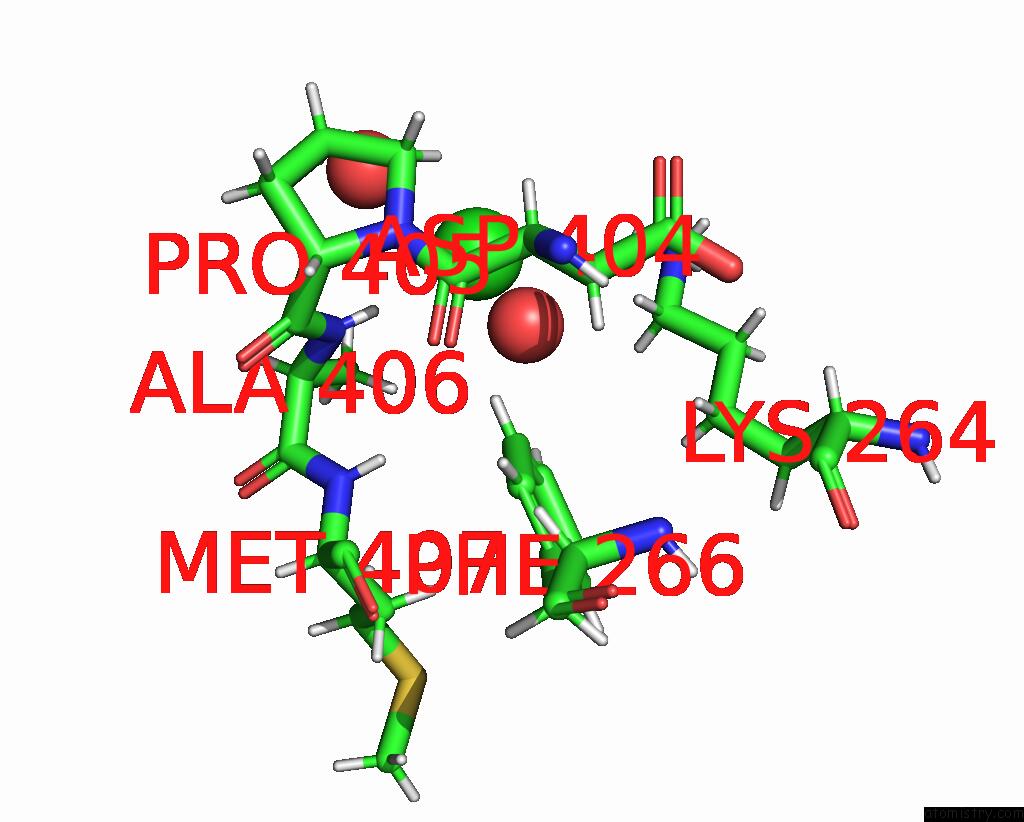
Mono view
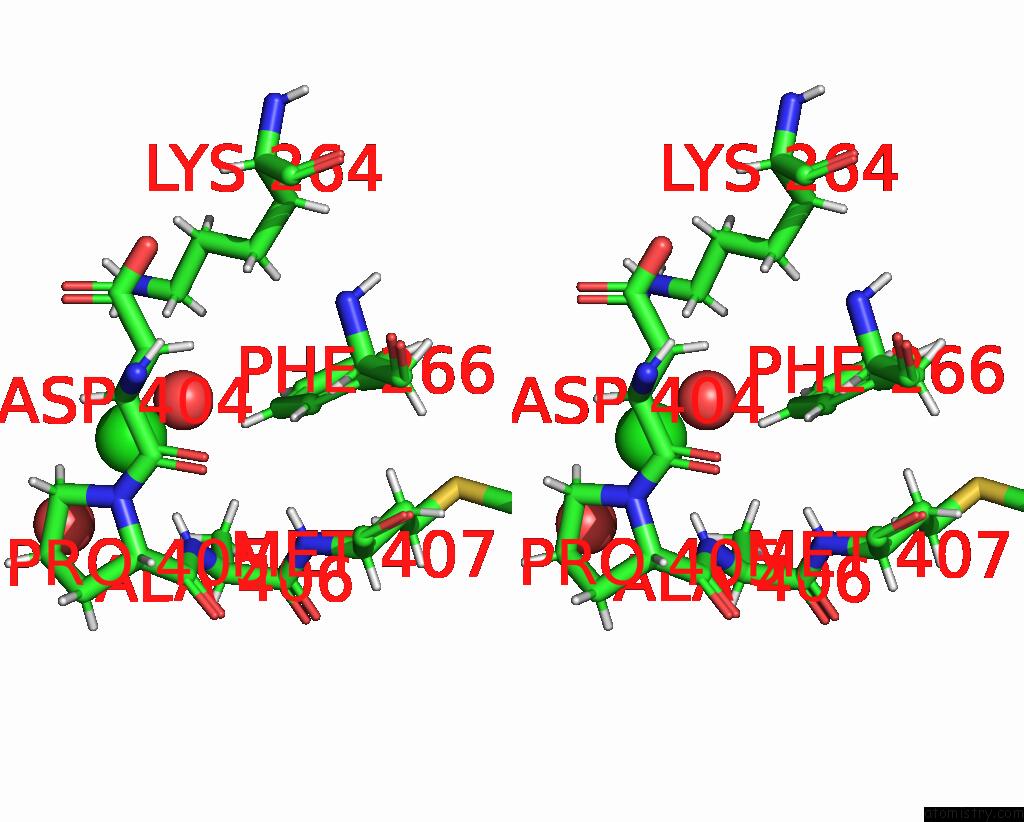
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Co-Bound [Fefe]-Hydrogenase I From Clostridium Pasteurianum (Cpi) within 5.0Å range:
|
Reference:
J.Duan,
A.Hemschemeier,
D.J.Burr,
S.T.Stripp,
E.Hofmann,
T.Happe.
Cyanide Binding to [Fefe]-Hydrogenase Stabilizes the Alternative Configuration of the Proton Transfer Pathway. Angew.Chem.Int.Ed.Engl. 2022.
ISSN: ESSN 1521-3773
PubMed: 36464641
DOI: 10.1002/ANIE.202216903
Page generated: Sun Jul 13 09:07:20 2025
ISSN: ESSN 1521-3773
PubMed: 36464641
DOI: 10.1002/ANIE.202216903
Last articles
F in 7MXGF in 7MXH
F in 7MX7
F in 7MVS
F in 7MX0
F in 7MXA
F in 7MT6
F in 7MSA
F in 7MTY
F in 7MT5