Chlorine »
PDB 8huw-8ig1 »
8ibl »
Chlorine in PDB 8ibl: Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation
Enzymatic activity of Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation
All present enzymatic activity of Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation:
3.1.1.74;
3.1.1.74;
Protein crystallography data
The structure of Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation, PDB code: 8ibl
was solved by
M.Emori,
N.Numoto,
N.Kamiya,
M.Oda,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 32.36 / 2.60 |
| Space group | P 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 83.869, 83.869, 64.723, 90, 90, 120 |
| R / Rfree (%) | 21.1 / 26.3 |
Other elements in 8ibl:
The structure of Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation also contains other interesting chemical elements:
| Calcium | (Ca) | 2 atoms |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation
(pdb code 8ibl). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 2 binding sites of Chlorine where determined in the Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation, PDB code: 8ibl:
Jump to Chlorine binding site number: 1; 2;
In total 2 binding sites of Chlorine where determined in the Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation, PDB code: 8ibl:
Jump to Chlorine binding site number: 1; 2;
Chlorine binding site 1 out of 2 in 8ibl
Go back to
Chlorine binding site 1 out
of 2 in the Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation within 5.0Å range:
|
Chlorine binding site 2 out of 2 in 8ibl
Go back to
Chlorine binding site 2 out
of 2 in the Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation

Mono view
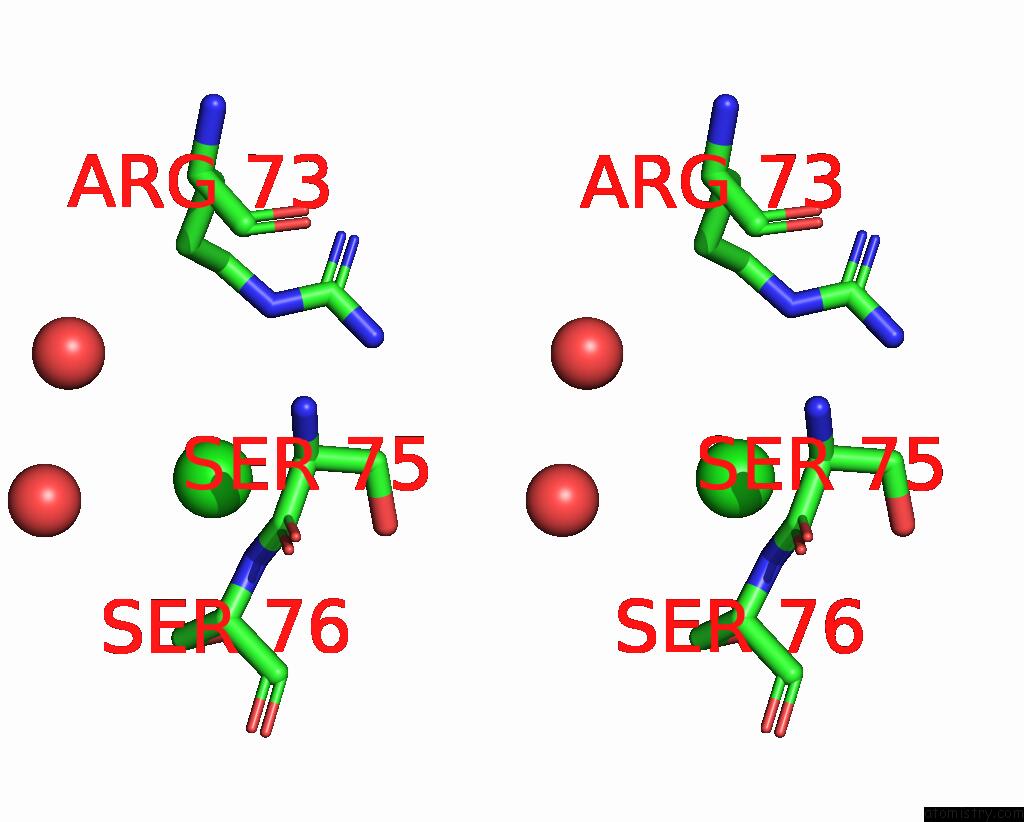
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Mes Bound Form of Pet-Degrading Cutinase CUT190 with Thermostability- Improving Mutations of S226P/R228S/Q138A/D250C-E296C/Q123H/N202H and S176A Inactivation within 5.0Å range:
|
Reference:
N.Numoto,
N.Kamiya,
M.Oda.
Improvement of Thermostability and Activity of Pet-Degrading Enzyme CUT190 Towards A Detailed Understanding and Application of the Enzymatic Reaction Mechanism. Biorxiv 2023.
ISSN: ISSN 2692-8205
DOI: 10.1101/2023.02.26.529345
Page generated: Sun Jul 13 12:12:47 2025
ISSN: ISSN 2692-8205
DOI: 10.1101/2023.02.26.529345
Last articles
F in 7QMOF in 7QMN
F in 7QMM
F in 7QML
F in 7QMK
F in 7QMJ
F in 7QMI
F in 7QMH
F in 7QMG
F in 7QMF