Chlorine »
PDB 9cr5-9dpx »
9dpt »
Chlorine in PDB 9dpt: Bmp-9 G389S Dimer with Radiation Damage in Neutral pH
Protein crystallography data
The structure of Bmp-9 G389S Dimer with Radiation Damage in Neutral pH, PDB code: 9dpt
was solved by
T.A.Schwartze,
A.P.Hinck,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.04 / 2.49 |
| Space group | I 41 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 70.771, 70.771, 145.523, 90, 90, 90 |
| R / Rfree (%) | 23.9 / 27.9 |
Other elements in 9dpt:
The structure of Bmp-9 G389S Dimer with Radiation Damage in Neutral pH also contains other interesting chemical elements:
| Sodium | (Na) | 4 atoms |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Bmp-9 G389S Dimer with Radiation Damage in Neutral pH
(pdb code 9dpt). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 5 binding sites of Chlorine where determined in the Bmp-9 G389S Dimer with Radiation Damage in Neutral pH, PDB code: 9dpt:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Chlorine where determined in the Bmp-9 G389S Dimer with Radiation Damage in Neutral pH, PDB code: 9dpt:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5;
Chlorine binding site 1 out of 5 in 9dpt
Go back to
Chlorine binding site 1 out
of 5 in the Bmp-9 G389S Dimer with Radiation Damage in Neutral pH
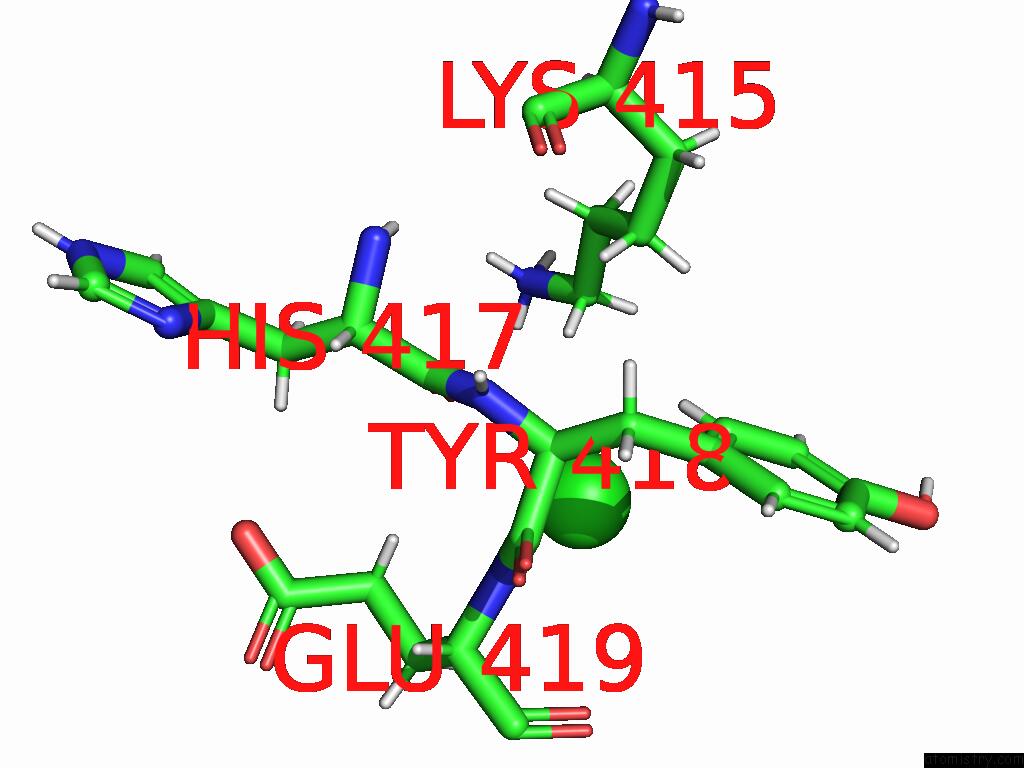
Mono view
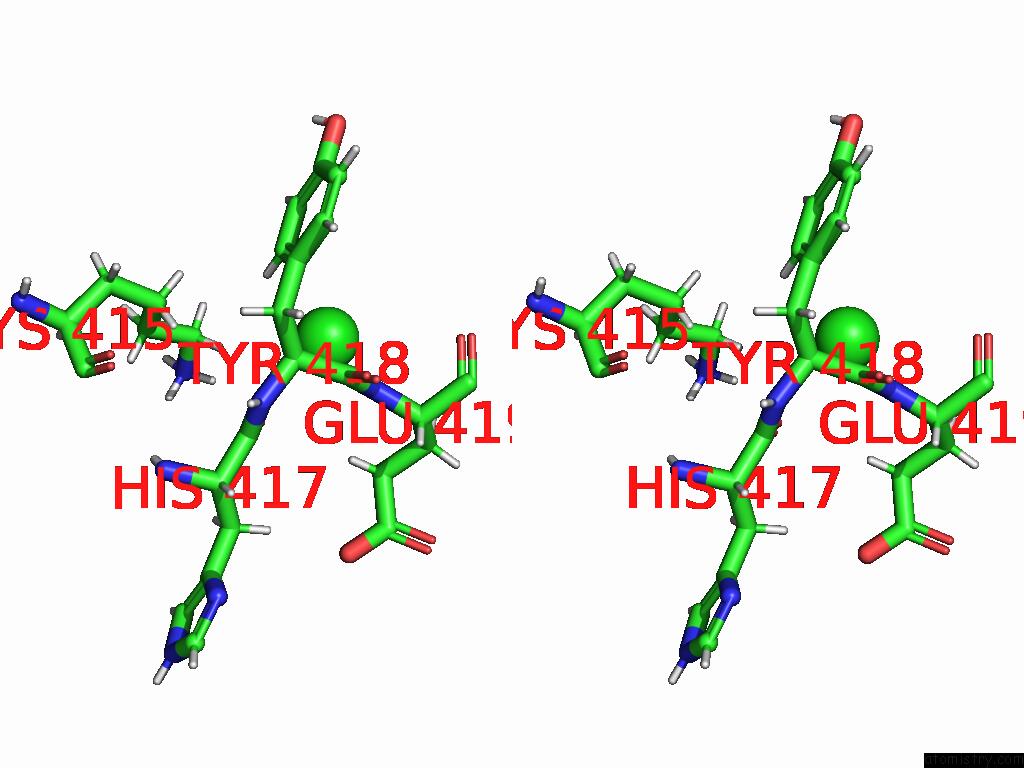
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Bmp-9 G389S Dimer with Radiation Damage in Neutral pH within 5.0Å range:
|
Chlorine binding site 2 out of 5 in 9dpt
Go back to
Chlorine binding site 2 out
of 5 in the Bmp-9 G389S Dimer with Radiation Damage in Neutral pH
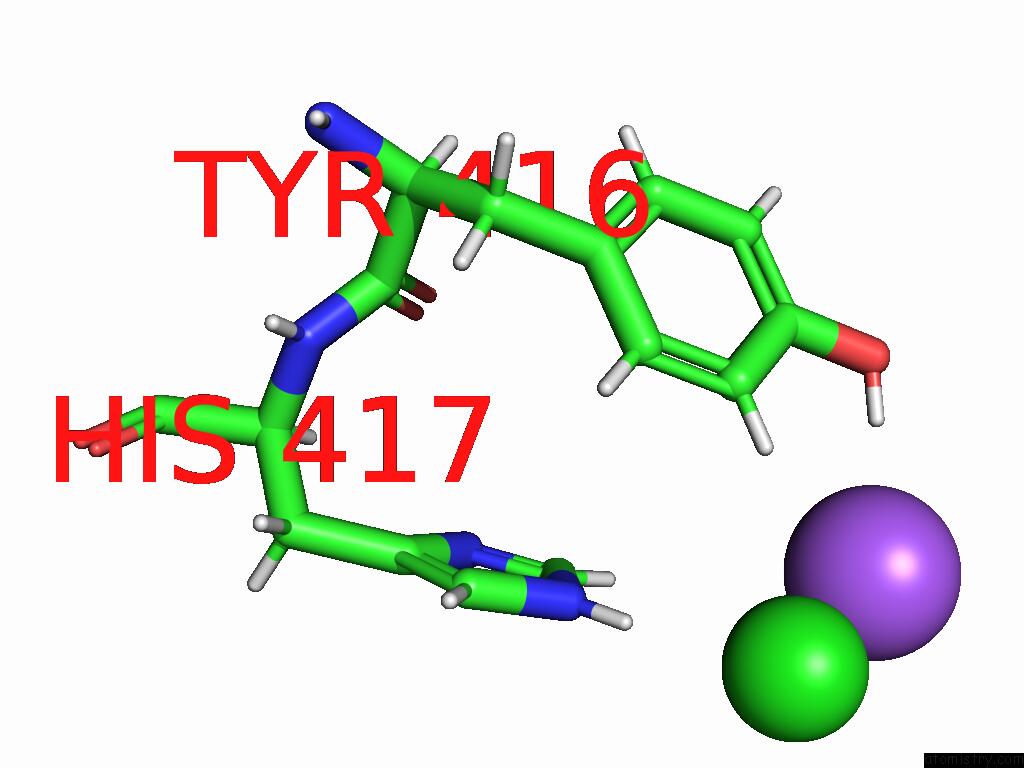
Mono view
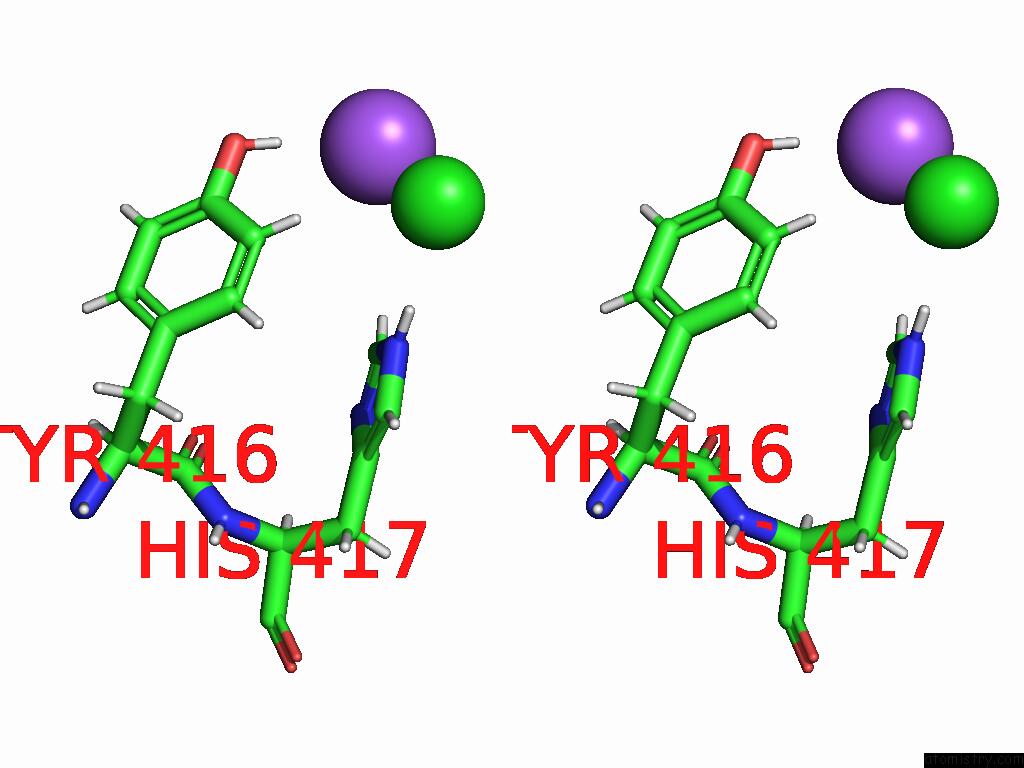
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Bmp-9 G389S Dimer with Radiation Damage in Neutral pH within 5.0Å range:
|
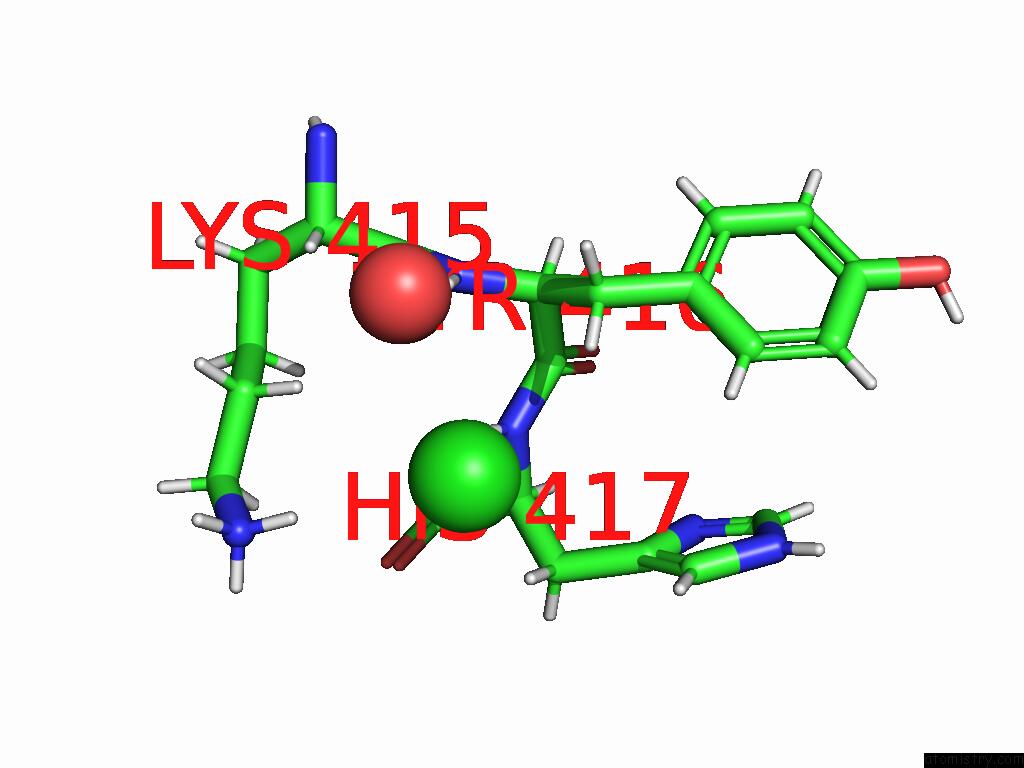
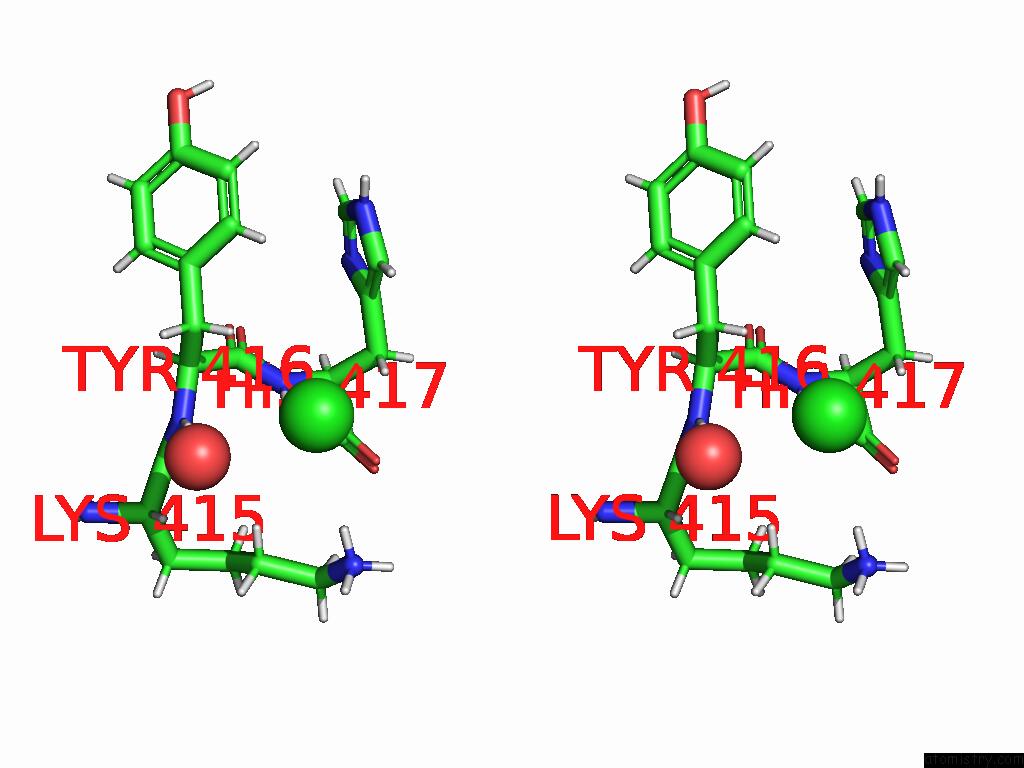
Chlorine binding site 3 out of 5 in 9dpt
Go back to
Chlorine binding site 3 out
of 5 in the Bmp-9 G389S Dimer with Radiation Damage in Neutral pH
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 3 of Bmp-9 G389S Dimer with Radiation Damage in Neutral pH within 5.0Å range:
|
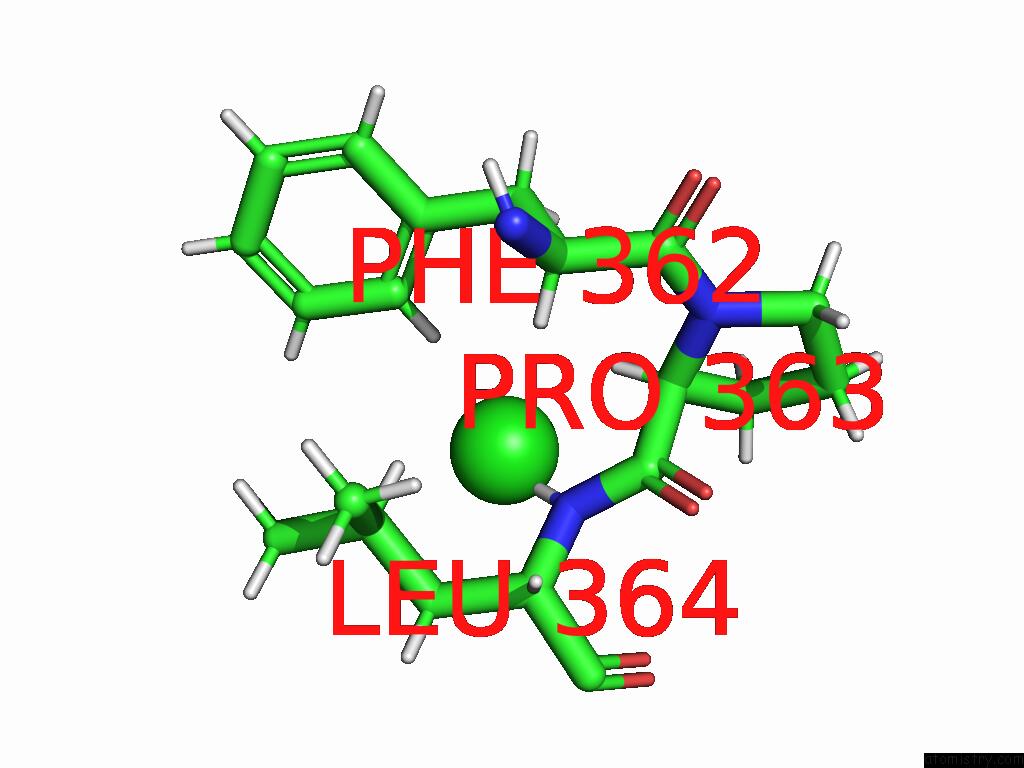
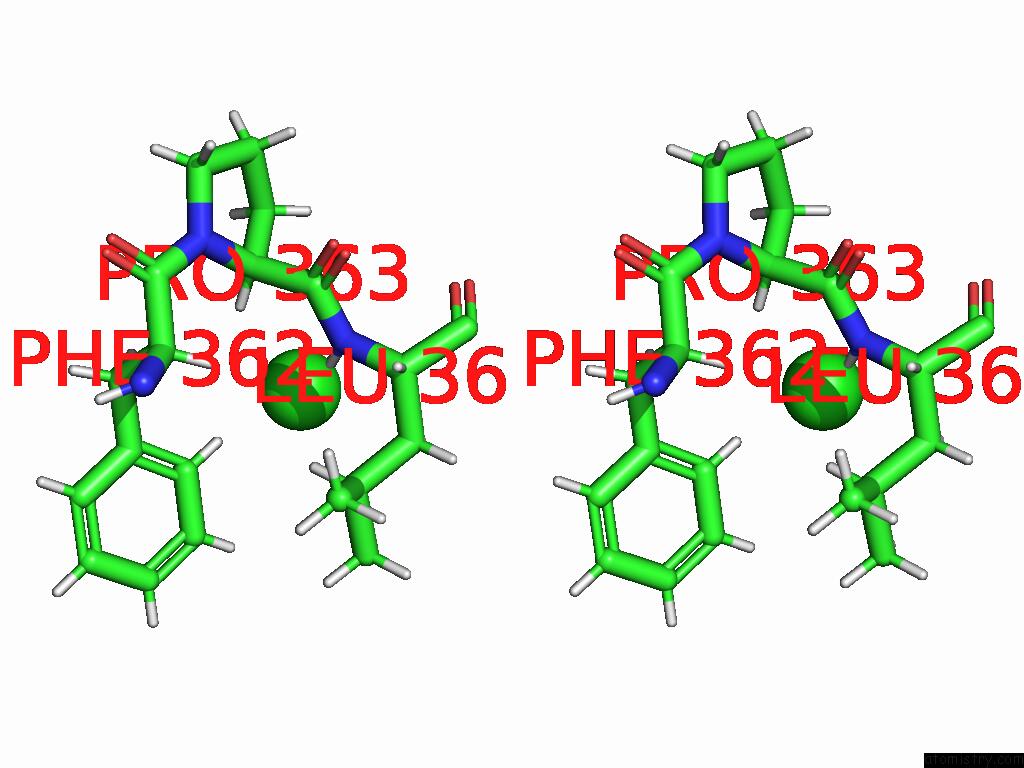
Chlorine binding site 4 out of 5 in 9dpt
Go back to
Chlorine binding site 4 out
of 5 in the Bmp-9 G389S Dimer with Radiation Damage in Neutral pH
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 4 of Bmp-9 G389S Dimer with Radiation Damage in Neutral pH within 5.0Å range:
|
Chlorine binding site 5 out of 5 in 9dpt
Go back to
Chlorine binding site 5 out
of 5 in the Bmp-9 G389S Dimer with Radiation Damage in Neutral pH
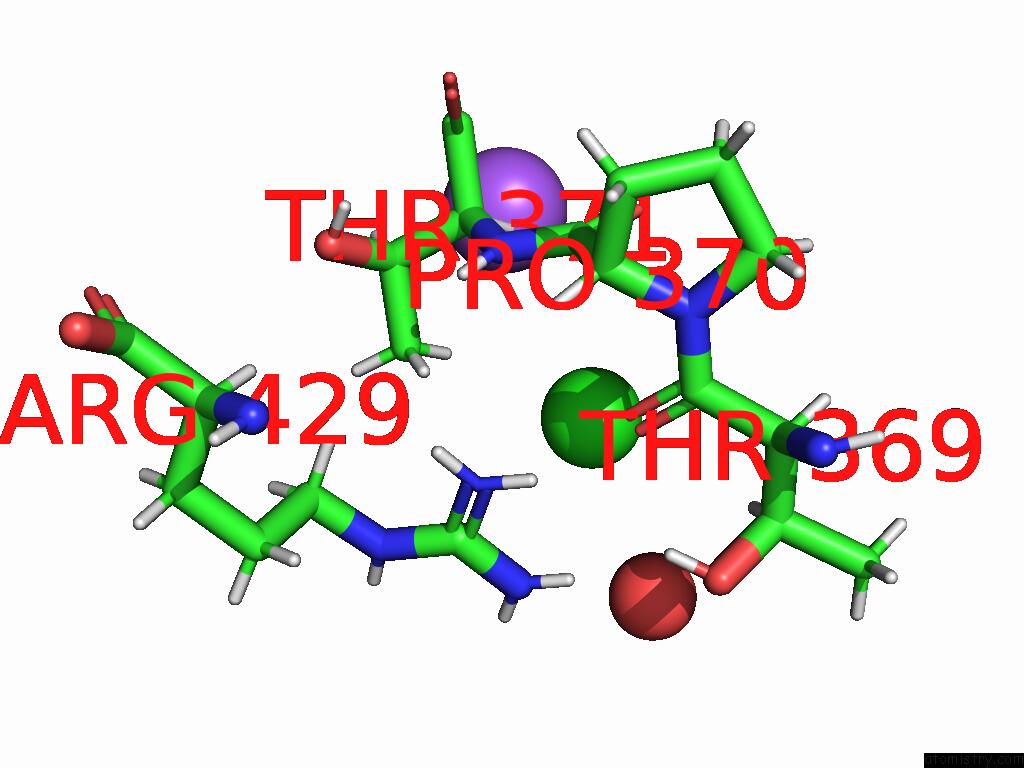
Mono view
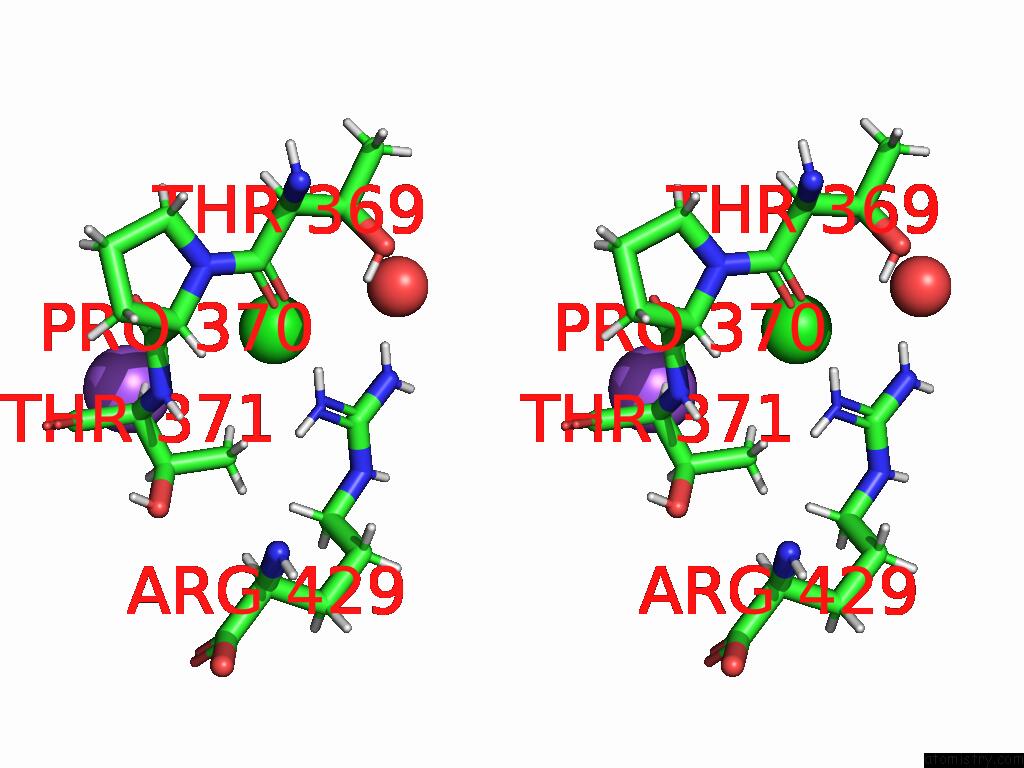
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 5 of Bmp-9 G389S Dimer with Radiation Damage in Neutral pH within 5.0Å range:
|
Reference:
T.A.Schwartze,
S.A.Morosky,
T.L.Rosato,
A.Henrickson,
G.Lin,
C.S.Hinck,
A.B.Taylor,
S.K.Olsen,
G.Calero,
B.Demeler,
B.L.Roman,
A.P.Hinck.
Molecular Basis of Interchain Disulfide Bond Formation in Bmp-9 and Bmp-10. J.Mol.Biol. V. 437 68935 2025.
ISSN: ESSN 1089-8638
PubMed: 39793884
DOI: 10.1016/J.JMB.2025.168935
Page generated: Sun Jul 13 16:21:42 2025
ISSN: ESSN 1089-8638
PubMed: 39793884
DOI: 10.1016/J.JMB.2025.168935
Last articles
Cu in 1BAWCu in 1BA9
Cu in 1B3I
Cu in 1B4T
Cu in 1AZR
Cu in 1B4L
Cu in 1AZV
Cu in 1AZU
Cu in 1AZN
Cu in 1ASP