Chlorine »
PDB 1ahz-1bxz »
1b6g »
Chlorine in PDB 1b6g: Haloalkane Dehalogenase at pH 5.0 Containing Chloride
Enzymatic activity of Haloalkane Dehalogenase at pH 5.0 Containing Chloride
All present enzymatic activity of Haloalkane Dehalogenase at pH 5.0 Containing Chloride:
3.8.1.5;
3.8.1.5;
Protein crystallography data
The structure of Haloalkane Dehalogenase at pH 5.0 Containing Chloride, PDB code: 1b6g
was solved by
I.S.Ridder,
H.J.Rozeboom,
B.W.Dijkstra,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 15.00 / 1.15 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 92.180, 72.029, 40.909, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 10.6 / 14.5 |
Other elements in 1b6g:
The structure of Haloalkane Dehalogenase at pH 5.0 Containing Chloride also contains other interesting chemical elements:
| Lead | (Pb) | 2 atoms |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Haloalkane Dehalogenase at pH 5.0 Containing Chloride
(pdb code 1b6g). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 2 binding sites of Chlorine where determined in the Haloalkane Dehalogenase at pH 5.0 Containing Chloride, PDB code: 1b6g:
Jump to Chlorine binding site number: 1; 2;
In total 2 binding sites of Chlorine where determined in the Haloalkane Dehalogenase at pH 5.0 Containing Chloride, PDB code: 1b6g:
Jump to Chlorine binding site number: 1; 2;
Chlorine binding site 1 out of 2 in 1b6g
Go back to
Chlorine binding site 1 out
of 2 in the Haloalkane Dehalogenase at pH 5.0 Containing Chloride
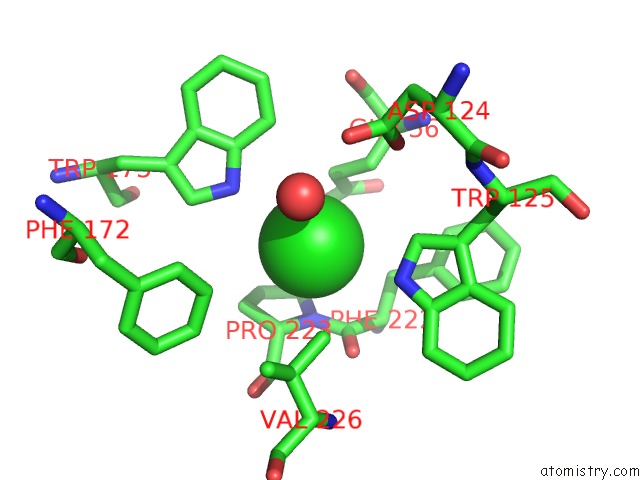
Mono view
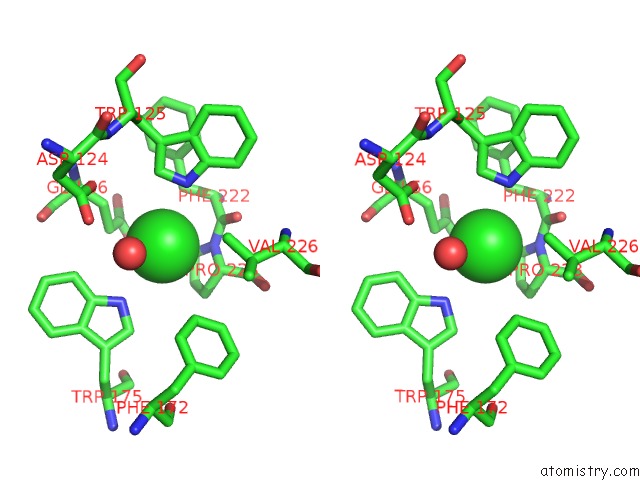
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Haloalkane Dehalogenase at pH 5.0 Containing Chloride within 5.0Å range:
|
Chlorine binding site 2 out of 2 in 1b6g
Go back to
Chlorine binding site 2 out
of 2 in the Haloalkane Dehalogenase at pH 5.0 Containing Chloride
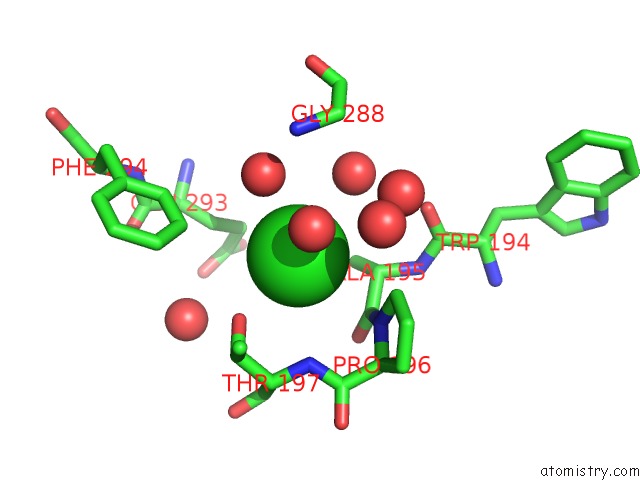
Mono view
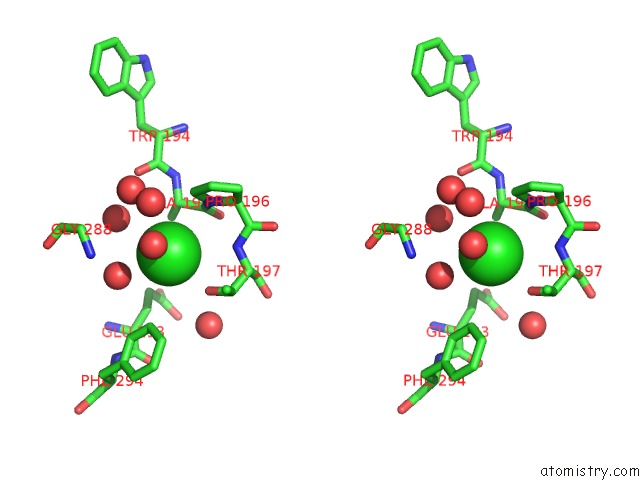
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Haloalkane Dehalogenase at pH 5.0 Containing Chloride within 5.0Å range:
|
Reference:
I.S.Ridder,
H.J.Rozeboom,
B.W.Dijkstra.
Haloalkane Dehalogenase From Xanthobacter Autotrophicus GJ10 Refined at 1.15 A Resolution. Acta Crystallogr.,Sect.D V. 55 1273 1999.
ISSN: ISSN 0907-4449
PubMed: 10393294
DOI: 10.1107/S090744499900534X
Page generated: Thu Jul 10 16:19:49 2025
ISSN: ISSN 0907-4449
PubMed: 10393294
DOI: 10.1107/S090744499900534X
Last articles
Mg in 2OQYMg in 2OUP
Mg in 2OUN
Mg in 2OU7
Mg in 2OTG
Mg in 2OSB
Mg in 2OS8
Mg in 2ORI
Mg in 2ORW
Mg in 2ONP