Chlorine »
PDB 3stf-3t4u »
3t4p »
Chlorine in PDB 3t4p: Crystal Structure of O-Acetyl Serine Sulfhydrylase From Leishmania Donovani in Complex with Designed Tetrapeptide
Enzymatic activity of Crystal Structure of O-Acetyl Serine Sulfhydrylase From Leishmania Donovani in Complex with Designed Tetrapeptide
All present enzymatic activity of Crystal Structure of O-Acetyl Serine Sulfhydrylase From Leishmania Donovani in Complex with Designed Tetrapeptide:
2.5.1.47;
2.5.1.47;
Protein crystallography data
The structure of Crystal Structure of O-Acetyl Serine Sulfhydrylase From Leishmania Donovani in Complex with Designed Tetrapeptide, PDB code: 3t4p
was solved by
I.Raj,
S.Gourinath,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 34.69 / 1.77 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 115.300, 61.930, 43.440, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.7 / 20.8 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Crystal Structure of O-Acetyl Serine Sulfhydrylase From Leishmania Donovani in Complex with Designed Tetrapeptide
(pdb code 3t4p). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the Crystal Structure of O-Acetyl Serine Sulfhydrylase From Leishmania Donovani in Complex with Designed Tetrapeptide, PDB code: 3t4p:
In total only one binding site of Chlorine was determined in the Crystal Structure of O-Acetyl Serine Sulfhydrylase From Leishmania Donovani in Complex with Designed Tetrapeptide, PDB code: 3t4p:
Chlorine binding site 1 out of 1 in 3t4p
Go back to
Chlorine binding site 1 out
of 1 in the Crystal Structure of O-Acetyl Serine Sulfhydrylase From Leishmania Donovani in Complex with Designed Tetrapeptide
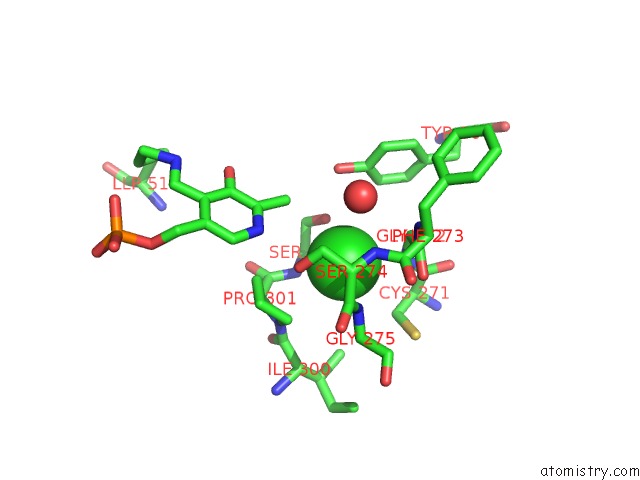
Mono view
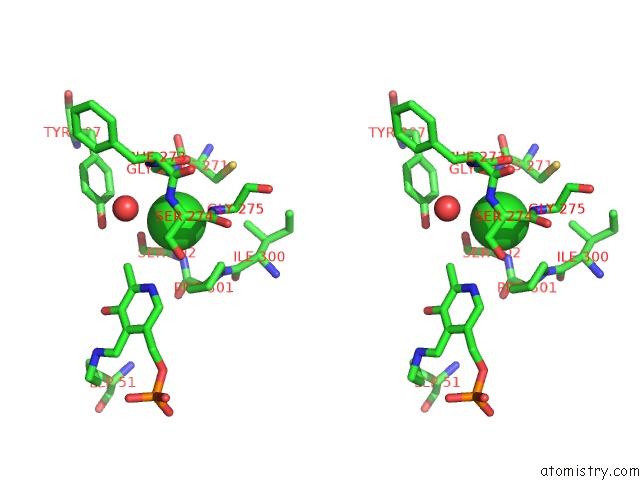
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Crystal Structure of O-Acetyl Serine Sulfhydrylase From Leishmania Donovani in Complex with Designed Tetrapeptide within 5.0Å range:
|
Reference:
I.Raj,
S.Kumar,
S.Gourinath.
The Narrow Active-Site Cleft of O-Acetylserine Sulfhydrylase From Leishmania Donovani Allows Complex Formation with Serine Acetyltransferases with A Range of C-Terminal Sequences Acta Crystallogr.,Sect.D V. 68 909 2012.
ISSN: ISSN 0907-4449
PubMed: 22868756
DOI: 10.1107/S0907444912016459
Page generated: Fri Jul 11 10:35:57 2025
ISSN: ISSN 0907-4449
PubMed: 22868756
DOI: 10.1107/S0907444912016459
Last articles
Mg in 4WF7Mg in 4WEC
Mg in 4WEO
Mg in 4WCW
Mg in 4WE1
Mg in 4WCU
Mg in 4WDQ
Mg in 4WB6
Mg in 4WB8
Mg in 4WC0