Chlorine »
PDB 3zx3-4a7i »
3zxd »
Chlorine in PDB 3zxd: Wild-Type Lysenin
Protein crystallography data
The structure of Wild-Type Lysenin, PDB code: 3zxd
was solved by
L.De Colibus,
A.F.P.Sonnen,
K.J.Morris,
C.A.Siebert,
P.Abrusci,
J.Plitzko,
V.Hodnik,
M.Leippe,
E.Volpi,
G.Anderluh,
R.J.C.Gilbert,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.09 / 3.30 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 58.910, 85.560, 108.810, 98.88, 96.84, 90.04 |
| R / Rfree (%) | 21.3 / 23.6 |
Other elements in 3zxd:
The structure of Wild-Type Lysenin also contains other interesting chemical elements:
| Magnesium | (Mg) | 6 atoms |
| Sodium | (Na) | 6 atoms |
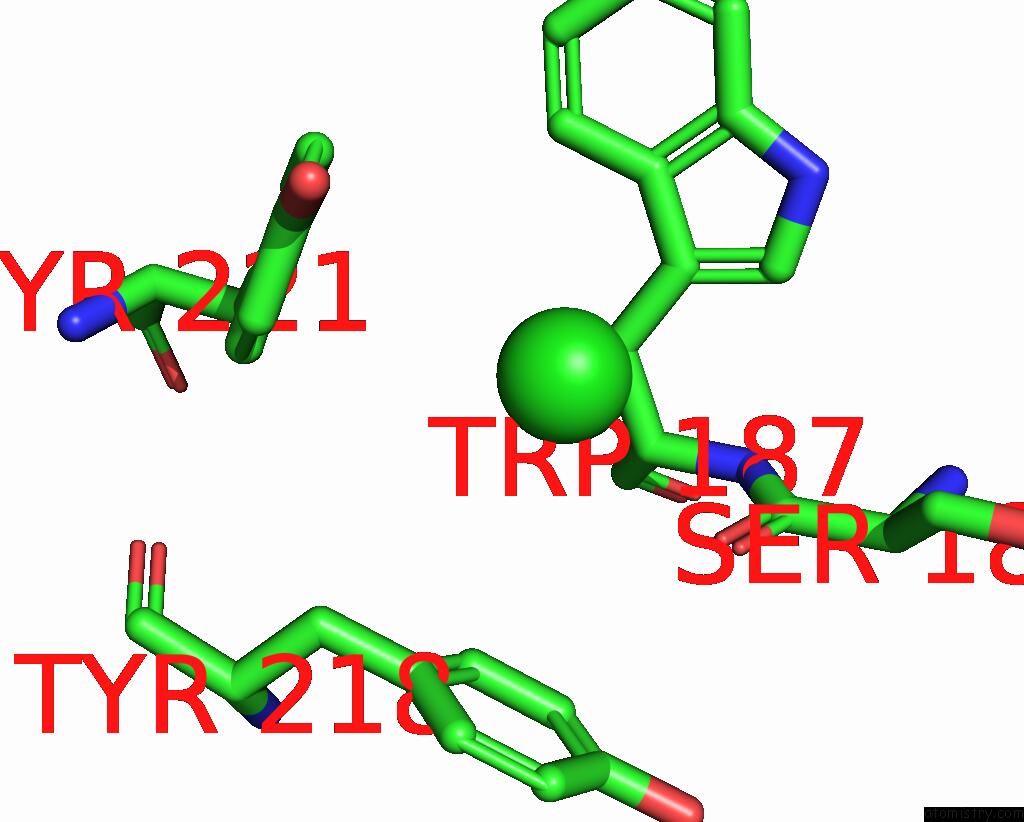
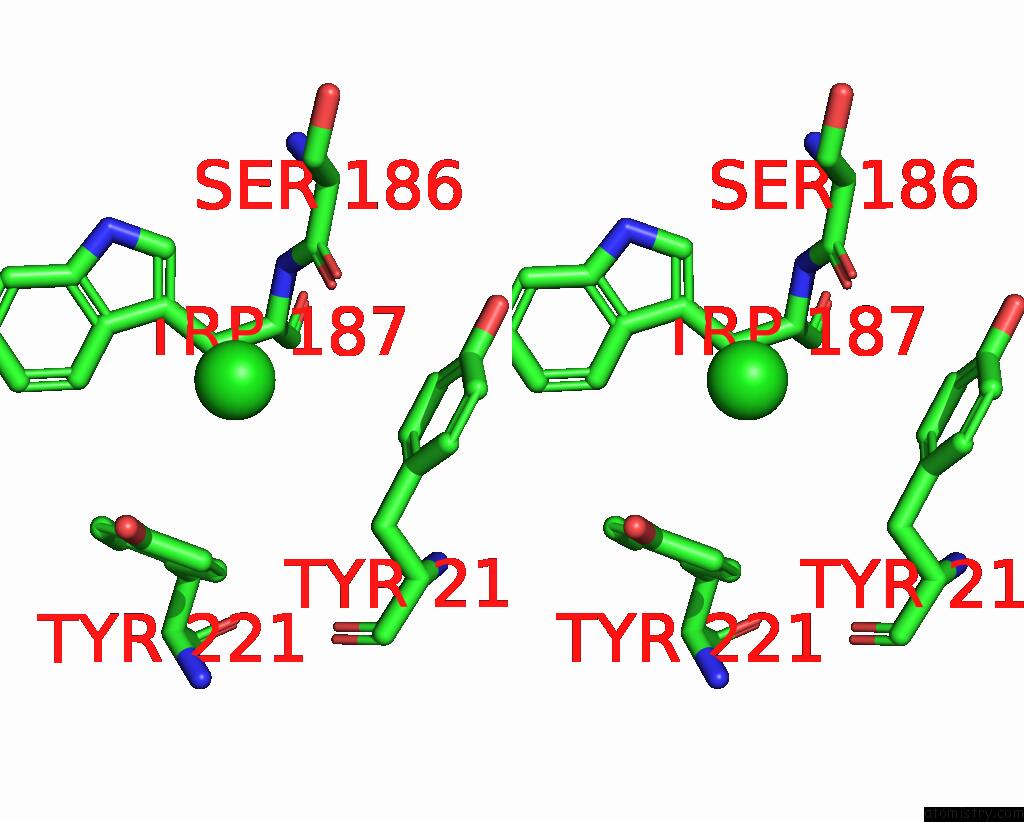
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Wild-Type Lysenin
(pdb code 3zxd). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the Wild-Type Lysenin, PDB code: 3zxd:
In total only one binding site of Chlorine was determined in the Wild-Type Lysenin, PDB code: 3zxd:
Chlorine binding site 1 out of 1 in 3zxd
Go back to
Chlorine binding site 1 out
of 1 in the Wild-Type Lysenin
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Wild-Type Lysenin within 5.0Å range:
|
Reference:
L.De Colibus,
A.F.P.Sonnen,
K.J.Morris,
C.A.Siebert,
P.Abrusci,
J.Plitzko,
V.Hodnik,
M.Leippe,
E.Volpi,
G.Anderluh,
R.J.C.Gilbert.
Structures of Lysenin Reveal A Shared Evolutionary Origin For Pore-Forming Proteins and Its Mode of Sphingomyelin Recognition. Structure V. 20 1498 2012.
ISSN: ISSN 0969-2126
PubMed: 22819216
DOI: 10.1016/J.STR.2012.06.011
Page generated: Fri Jul 11 12:35:10 2025
ISSN: ISSN 0969-2126
PubMed: 22819216
DOI: 10.1016/J.STR.2012.06.011
Last articles
K in 7P2SK in 7OX9
K in 7P1Z
K in 7OY3
K in 7OZZ
K in 7OX7
K in 7OX8
K in 7OV7
K in 7OTJ
K in 7OTB