Chlorine »
PDB 4rjt-4rsd »
4rrm »
Chlorine in PDB 4rrm: E129A Mutant of N-Terminal Editing Domain of Threonyl-Trna Synthetase From Aeropyrum Pernix with L-THR3AA
Enzymatic activity of E129A Mutant of N-Terminal Editing Domain of Threonyl-Trna Synthetase From Aeropyrum Pernix with L-THR3AA
All present enzymatic activity of E129A Mutant of N-Terminal Editing Domain of Threonyl-Trna Synthetase From Aeropyrum Pernix with L-THR3AA:
6.1.1.3;
6.1.1.3;
Protein crystallography data
The structure of E129A Mutant of N-Terminal Editing Domain of Threonyl-Trna Synthetase From Aeropyrum Pernix with L-THR3AA, PDB code: 4rrm
was solved by
S.Ahmad,
S.Muthukumar,
R.Sankaranarayanan,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 25.00 / 1.55 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 47.091, 47.091, 112.729, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.8 / 25.9 |
Other elements in 4rrm:
The structure of E129A Mutant of N-Terminal Editing Domain of Threonyl-Trna Synthetase From Aeropyrum Pernix with L-THR3AA also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the E129A Mutant of N-Terminal Editing Domain of Threonyl-Trna Synthetase From Aeropyrum Pernix with L-THR3AA
(pdb code 4rrm). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the E129A Mutant of N-Terminal Editing Domain of Threonyl-Trna Synthetase From Aeropyrum Pernix with L-THR3AA, PDB code: 4rrm:
In total only one binding site of Chlorine was determined in the E129A Mutant of N-Terminal Editing Domain of Threonyl-Trna Synthetase From Aeropyrum Pernix with L-THR3AA, PDB code: 4rrm:
Chlorine binding site 1 out of 1 in 4rrm
Go back to
Chlorine binding site 1 out
of 1 in the E129A Mutant of N-Terminal Editing Domain of Threonyl-Trna Synthetase From Aeropyrum Pernix with L-THR3AA
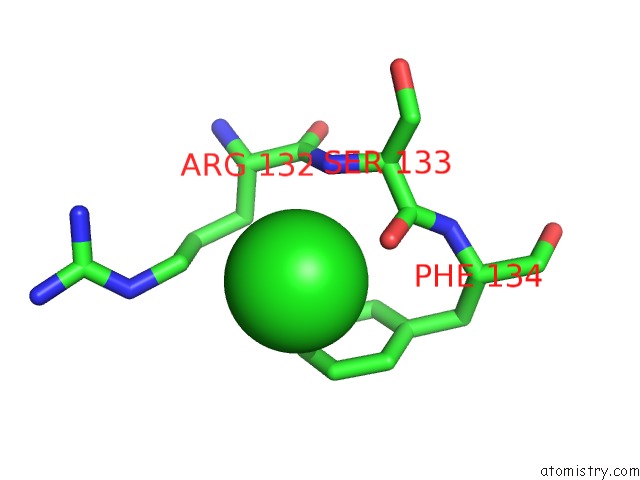
Mono view
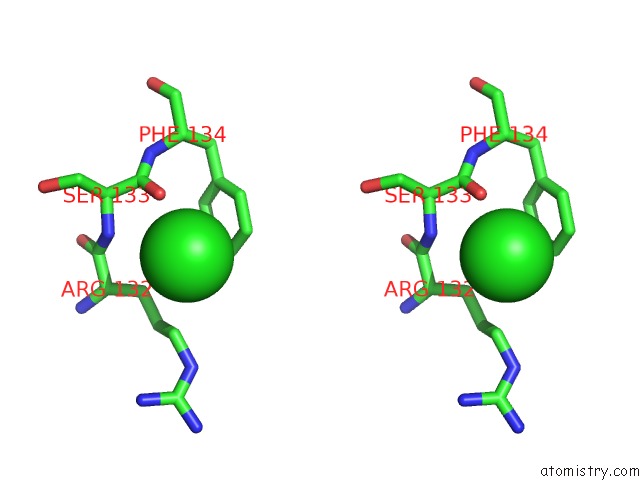
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of E129A Mutant of N-Terminal Editing Domain of Threonyl-Trna Synthetase From Aeropyrum Pernix with L-THR3AA within 5.0Å range:
|
Reference:
S.Ahmad,
S.Muthukumar,
S.K.Kuncha,
S.B.Routh,
A.S.Yerabham,
T.Hussain,
V.Kamarthapu,
S.P.Kruparani,
R.Sankaranarayanan.
Specificity and Catalysis Hardwired at the Rna-Protein Interface in A Translational Proofreading Enzyme. Nat Commun V. 6 7552 2015.
ISSN: ESSN 2041-1723
PubMed: 26113036
DOI: 10.1038/NCOMMS8552
Page generated: Fri Jul 11 21:31:25 2025
ISSN: ESSN 2041-1723
PubMed: 26113036
DOI: 10.1038/NCOMMS8552
Last articles
Zn in 3HUDZn in 3HSV
Zn in 3HTR
Zn in 3HT2
Zn in 3HTK
Zn in 3HRU
Zn in 3HSO
Zn in 3HSU
Zn in 3HSP
Zn in 3HSN