Chlorine »
PDB 5bvg-5c2c »
5c1c »
Chlorine in PDB 5c1c: Crystal Structure of the Pectin Methylesterase From Aspergillus Niger in Deglycosylated Form
Enzymatic activity of Crystal Structure of the Pectin Methylesterase From Aspergillus Niger in Deglycosylated Form
All present enzymatic activity of Crystal Structure of the Pectin Methylesterase From Aspergillus Niger in Deglycosylated Form:
3.1.1.11;
3.1.1.11;
Protein crystallography data
The structure of Crystal Structure of the Pectin Methylesterase From Aspergillus Niger in Deglycosylated Form, PDB code: 5c1c
was solved by
G.B.Jameson,
M.A.K.Williams,
T.S.Loo,
L.M.Kent,
L.D.Melton,
D.Mercadante,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.23 / 1.80 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 75.249, 113.843, 88.741, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17 / 20.3 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Crystal Structure of the Pectin Methylesterase From Aspergillus Niger in Deglycosylated Form
(pdb code 5c1c). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the Crystal Structure of the Pectin Methylesterase From Aspergillus Niger in Deglycosylated Form, PDB code: 5c1c:
In total only one binding site of Chlorine was determined in the Crystal Structure of the Pectin Methylesterase From Aspergillus Niger in Deglycosylated Form, PDB code: 5c1c:
Chlorine binding site 1 out of 1 in 5c1c
Go back to
Chlorine binding site 1 out
of 1 in the Crystal Structure of the Pectin Methylesterase From Aspergillus Niger in Deglycosylated Form
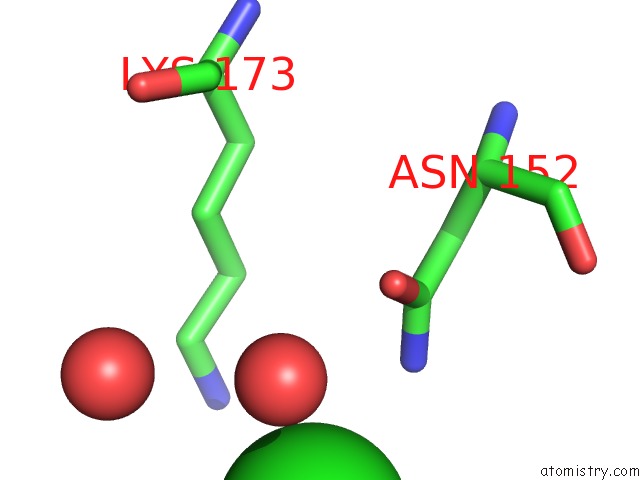
Mono view
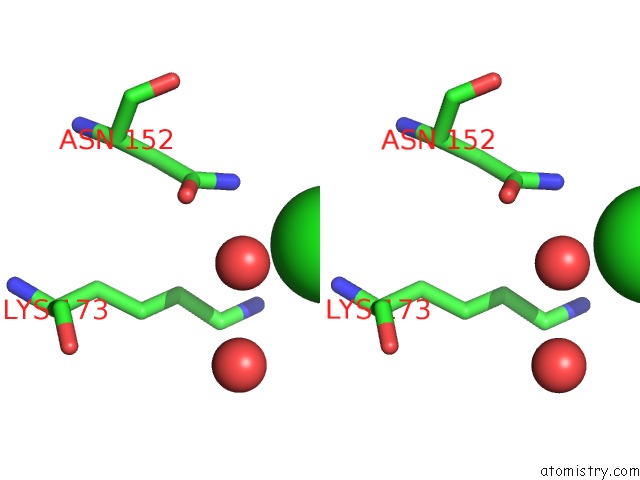
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Crystal Structure of the Pectin Methylesterase From Aspergillus Niger in Deglycosylated Form within 5.0Å range:
|
Reference:
L.M.Kent,
T.S.Loo,
L.D.Melton,
D.Mercadante,
M.A.Williams,
G.B.Jameson.
Structure and Properties of A Non-Processive, Salt-Requiring, and Acidophilic Pectin Methylesterase From Aspergillus Niger Provide Insights Into the Key Determinants of Processivity Control. J.Biol.Chem. V. 291 1289 2016.
ISSN: ESSN 1083-351X
PubMed: 26567911
DOI: 10.1074/JBC.M115.673152
Page generated: Sat Jul 12 00:37:43 2025
ISSN: ESSN 1083-351X
PubMed: 26567911
DOI: 10.1074/JBC.M115.673152
Last articles
Mn in 9LJUMn in 9LJW
Mn in 9LJS
Mn in 9LJR
Mn in 9LJT
Mn in 9LJV
Mg in 9UA2
Mg in 9R96
Mg in 9VM1
Mg in 9P01