Chlorine »
PDB 5nz7-5o5t »
5o58 »
Chlorine in PDB 5o58: Structure of the Inactive T.Maritima Pde (TM1595) D80N D154N Mutant with Substrate 5'-Papg
Protein crystallography data
The structure of Structure of the Inactive T.Maritima Pde (TM1595) D80N D154N Mutant with Substrate 5'-Papg, PDB code: 5o58
was solved by
G.Witte,
D.Drexler,
M.Mueller,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.33 / 1.55 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 70.370, 88.340, 124.270, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 15.8 / 18.4 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Structure of the Inactive T.Maritima Pde (TM1595) D80N D154N Mutant with Substrate 5'-Papg
(pdb code 5o58). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 2 binding sites of Chlorine where determined in the Structure of the Inactive T.Maritima Pde (TM1595) D80N D154N Mutant with Substrate 5'-Papg, PDB code: 5o58:
Jump to Chlorine binding site number: 1; 2;
In total 2 binding sites of Chlorine where determined in the Structure of the Inactive T.Maritima Pde (TM1595) D80N D154N Mutant with Substrate 5'-Papg, PDB code: 5o58:
Jump to Chlorine binding site number: 1; 2;
Chlorine binding site 1 out of 2 in 5o58
Go back to
Chlorine binding site 1 out
of 2 in the Structure of the Inactive T.Maritima Pde (TM1595) D80N D154N Mutant with Substrate 5'-Papg
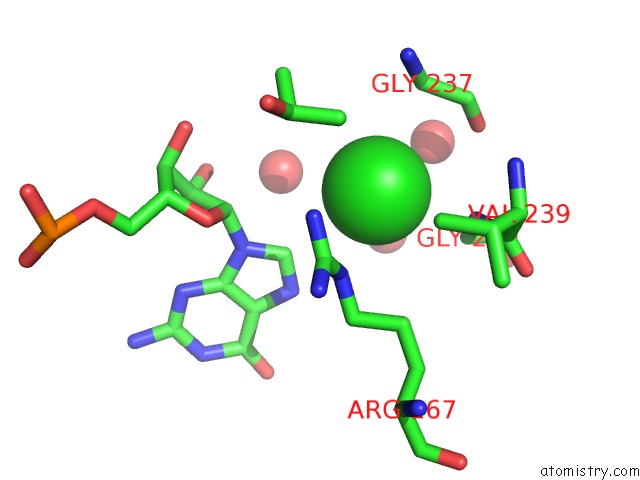
Mono view
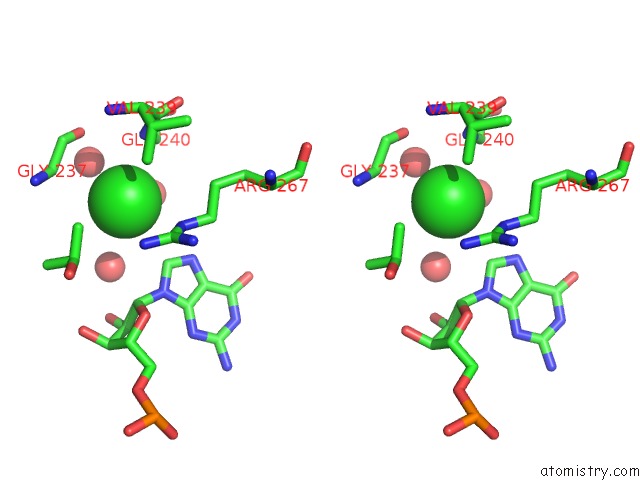
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Structure of the Inactive T.Maritima Pde (TM1595) D80N D154N Mutant with Substrate 5'-Papg within 5.0Å range:
|
Chlorine binding site 2 out of 2 in 5o58
Go back to
Chlorine binding site 2 out
of 2 in the Structure of the Inactive T.Maritima Pde (TM1595) D80N D154N Mutant with Substrate 5'-Papg
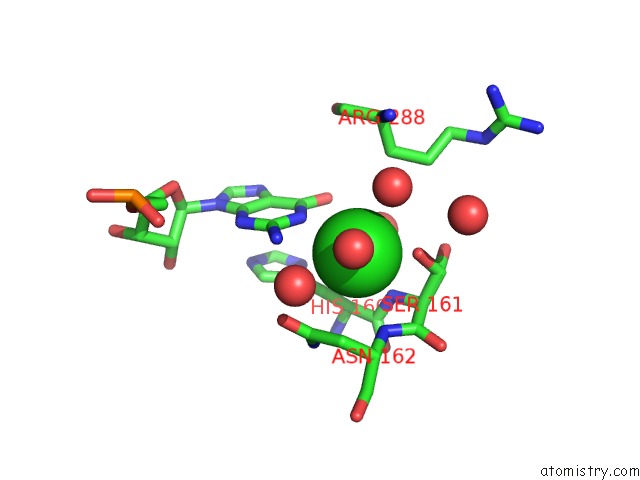
Mono view
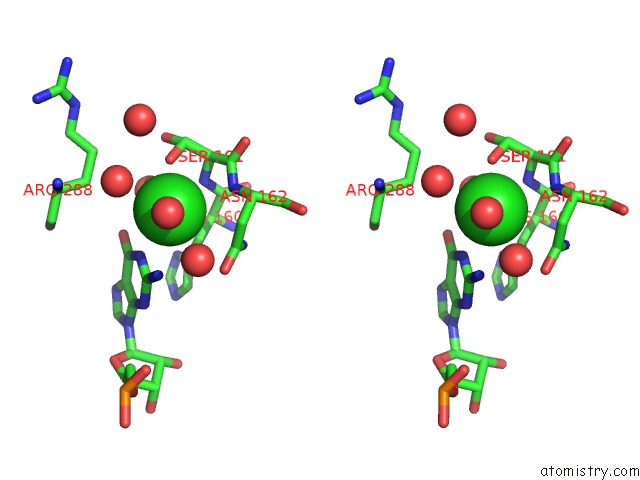
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Structure of the Inactive T.Maritima Pde (TM1595) D80N D154N Mutant with Substrate 5'-Papg within 5.0Å range:
|
Reference:
D.J.Drexler,
M.Muller,
C.A.Rojas-Cordova,
A.M.Bandera,
G.Witte.
Structural and Biophysical Analysis of the Soluble Dhh/DHHA1-Type Phosphodiesterase TM1595 From Thermotoga Maritima. Structure V. 25 1887 2017.
ISSN: ISSN 1878-4186
PubMed: 29107484
DOI: 10.1016/J.STR.2017.10.001
Page generated: Sat Jul 12 06:32:17 2025
ISSN: ISSN 1878-4186
PubMed: 29107484
DOI: 10.1016/J.STR.2017.10.001
Last articles
Mg in 8CGUMg in 8CGD
Mg in 8CGR
Mg in 8CEU
Mg in 8CAM
Mg in 8CGJ
Mg in 8CGI
Mg in 8CF8
Mg in 8CGA
Mg in 8CEP