Chlorine »
PDB 8rj2-8rwz »
8rrk »
Chlorine in PDB 8rrk: 14-3-3 Sigma Complexed with An Optimized Phosphopeptide
Protein crystallography data
The structure of 14-3-3 Sigma Complexed with An Optimized Phosphopeptide, PDB code: 8rrk
was solved by
A.Cousido-Siah,
A.G.Mcewen,
E.Monsellier,
G.Trave,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.49 / 1.93 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 138.25, 54.88, 82.12, 90, 101.27, 90 |
| R / Rfree (%) | 18 / 21.8 |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the 14-3-3 Sigma Complexed with An Optimized Phosphopeptide
(pdb code 8rrk). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total only one binding site of Chlorine was determined in the 14-3-3 Sigma Complexed with An Optimized Phosphopeptide, PDB code: 8rrk:
In total only one binding site of Chlorine was determined in the 14-3-3 Sigma Complexed with An Optimized Phosphopeptide, PDB code: 8rrk:
Chlorine binding site 1 out of 1 in 8rrk
Go back to
Chlorine binding site 1 out
of 1 in the 14-3-3 Sigma Complexed with An Optimized Phosphopeptide
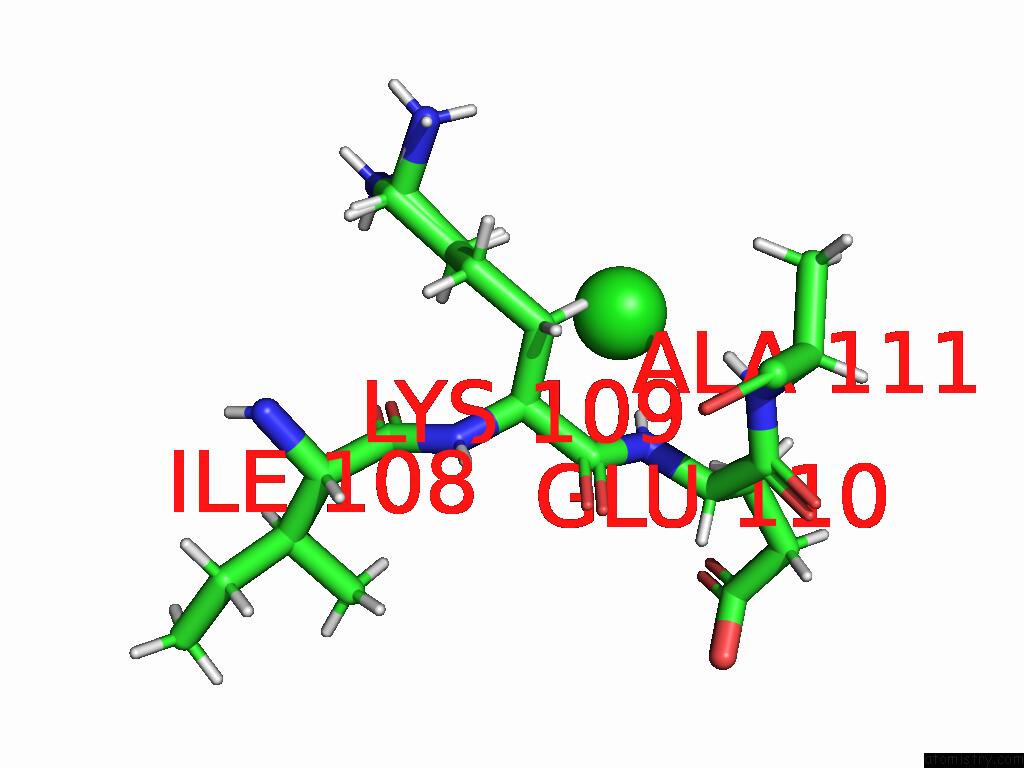
Mono view
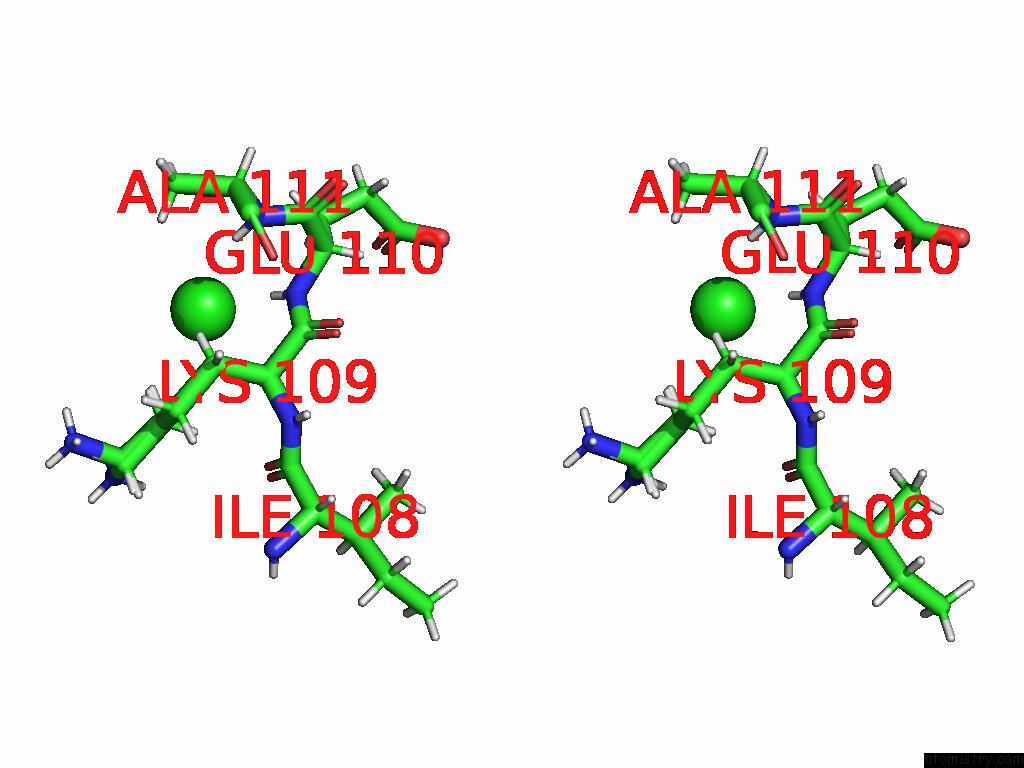
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of 14-3-3 Sigma Complexed with An Optimized Phosphopeptide within 5.0Å range:
|
Reference:
F.Delalande,
G.Gogl,
A.Cousido-Siah,
A.G.Mcewen,
A.Rohrbacher,
C.Kostmann,
Y.Nomine,
P.Eberling,
C.Carapito,
G.Trave,
E.Monsellier.
Holdup Multiplex Assay For High-Throughput Measurement of Protein-Ligand Affinity Constants Using A Mass-Spectrometry Readout To Be Published.
Page generated: Sun Jul 13 13:59:44 2025
Last articles
K in 7QQXK in 7QQW
K in 7QQU
K in 7QQV
K in 7QQT
K in 7QQR
K in 7QQS
K in 7QQQ
K in 7QQP
K in 7QQO