Chlorine »
PDB 4m5h-4mdq »
4ma9 »
Chlorine in PDB 4ma9: Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation
Enzymatic activity of Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation
All present enzymatic activity of Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation:
1.11.1.15;
1.11.1.15;
Protein crystallography data
The structure of Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation, PDB code: 4ma9
was solved by
A.Perkins,
P.A.Karplus,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.76 / 1.82 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 126.840, 171.150, 135.320, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.4 / 24 |
Other elements in 4ma9:
The structure of Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation also contains other interesting chemical elements:
| Potassium | (K) | 3 atoms |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation
(pdb code 4ma9). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 5 binding sites of Chlorine where determined in the Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation, PDB code: 4ma9:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Chlorine where determined in the Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation, PDB code: 4ma9:
Jump to Chlorine binding site number: 1; 2; 3; 4; 5;
Chlorine binding site 1 out of 5 in 4ma9
Go back to
Chlorine binding site 1 out
of 5 in the Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation
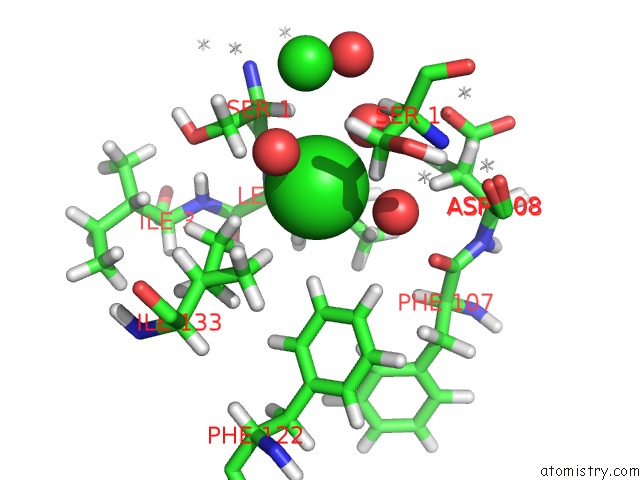
Mono view
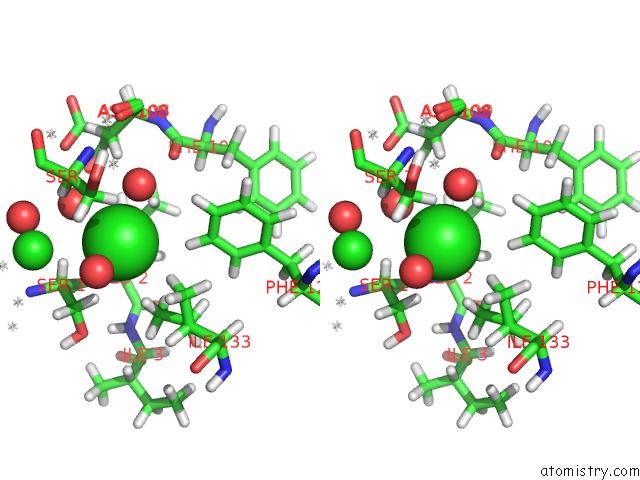
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation within 5.0Å range:
|
Chlorine binding site 2 out of 5 in 4ma9
Go back to
Chlorine binding site 2 out
of 5 in the Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation
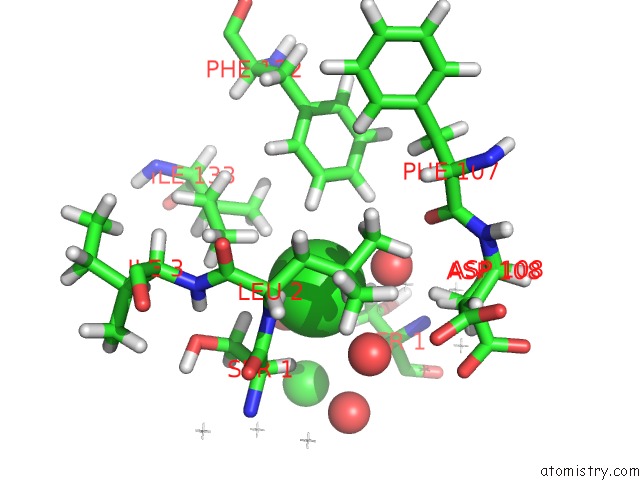
Mono view
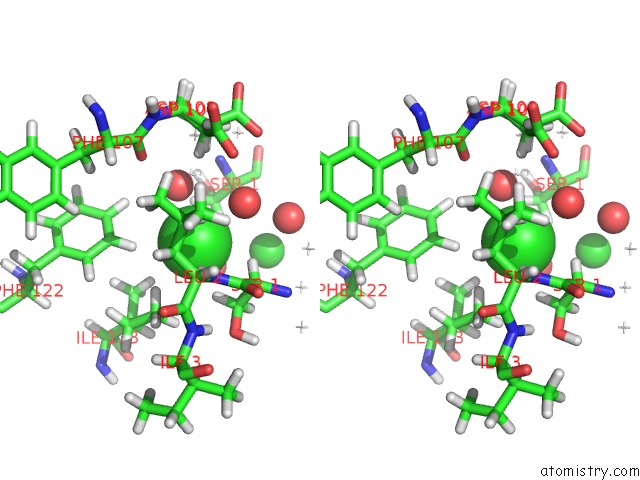
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation within 5.0Å range:
|
Chlorine binding site 3 out of 5 in 4ma9
Go back to
Chlorine binding site 3 out
of 5 in the Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation
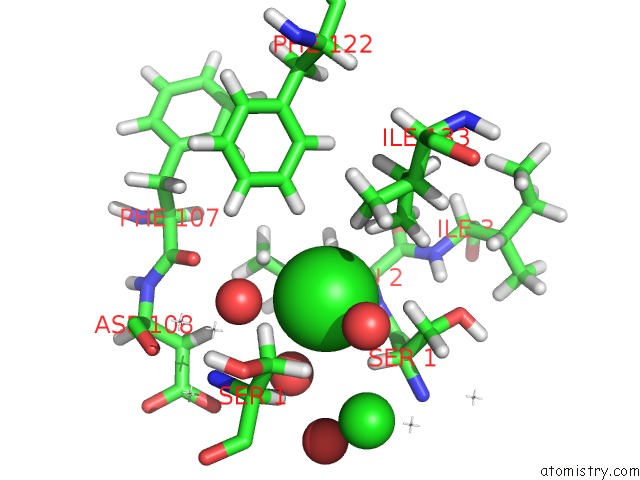
Mono view
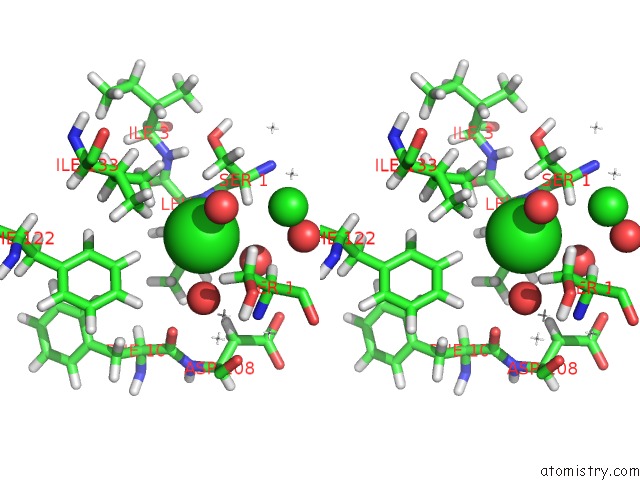
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 3 of Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation within 5.0Å range:
|
Chlorine binding site 4 out of 5 in 4ma9
Go back to
Chlorine binding site 4 out
of 5 in the Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation
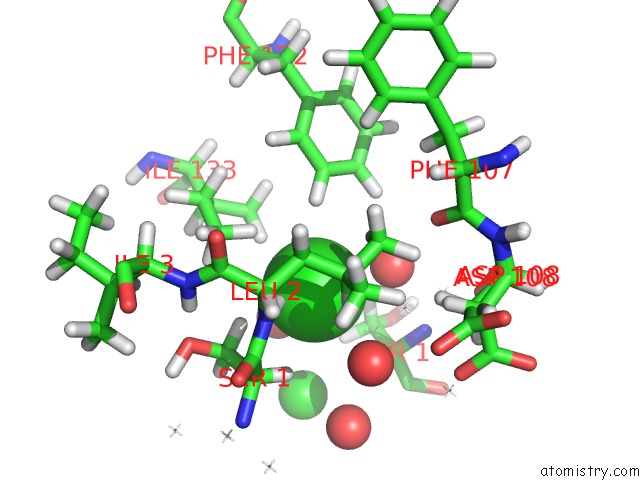
Mono view
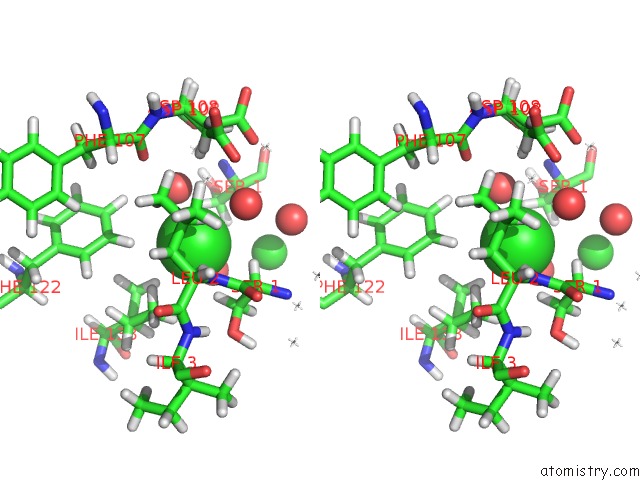
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 4 of Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation within 5.0Å range:
|
Chlorine binding site 5 out of 5 in 4ma9
Go back to
Chlorine binding site 5 out
of 5 in the Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation
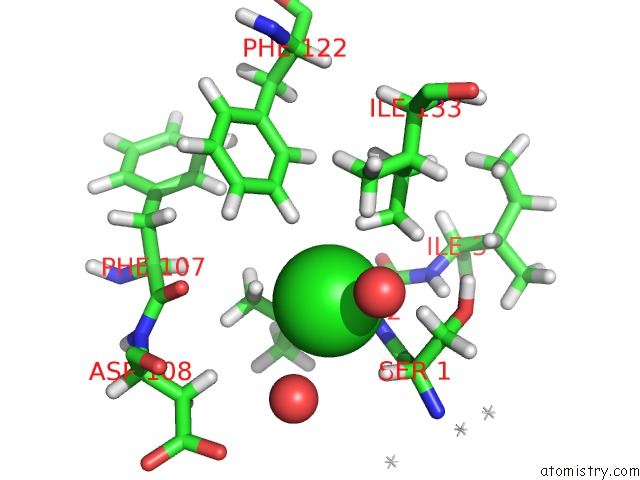
Mono view
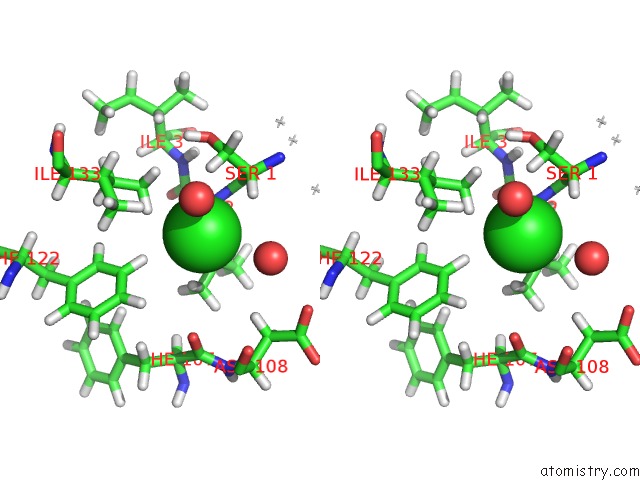
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 5 of Wild Type Salmonella Alkyl Hydroperoxide Reductase C in Its Substrate- Ready Conformation within 5.0Å range:
|
Reference:
A.Perkins,
K.J.Nelson,
J.R.Williams,
D.Parsonage,
L.B.Poole,
P.A.Karplus.
The Sensitive Balance Between the Fully Folded and Locally Unfolded Conformations of A Model Peroxiredoxin. Biochemistry V. 52 8708 2013.
ISSN: ISSN 0006-2960
PubMed: 24175952
DOI: 10.1021/BI4011573
Page generated: Sun Jul 21 19:38:42 2024
ISSN: ISSN 0006-2960
PubMed: 24175952
DOI: 10.1021/BI4011573
Last articles
Cl in 2WU6Cl in 2WTV
Cl in 2WTP
Cl in 2WTW
Cl in 2WTO
Cl in 2WRT
Cl in 2WTU
Cl in 2WQL
Cl in 2WTA
Cl in 2WSM