Chlorine »
PDB 4b4j-4bbp »
4b6z »
Chlorine in PDB 4b6z: Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia
Protein crystallography data
The structure of Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia, PDB code: 4b6z
was solved by
V.Rimsa,
T.C.Eadsforth,
R.P.Joosten,
W.N.Hunter,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 1.90 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 62.897, 85.947, 289.016, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.439 / 20.455 |
Other elements in 4b6z:
The structure of Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia also contains other interesting chemical elements:
| Zinc | (Zn) | 4 atoms |
Chlorine Binding Sites:
The binding sites of Chlorine atom in the Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia
(pdb code 4b6z). This binding sites where shown within
5.0 Angstroms radius around Chlorine atom.
In total 3 binding sites of Chlorine where determined in the Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia, PDB code: 4b6z:
Jump to Chlorine binding site number: 1; 2; 3;
In total 3 binding sites of Chlorine where determined in the Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia, PDB code: 4b6z:
Jump to Chlorine binding site number: 1; 2; 3;
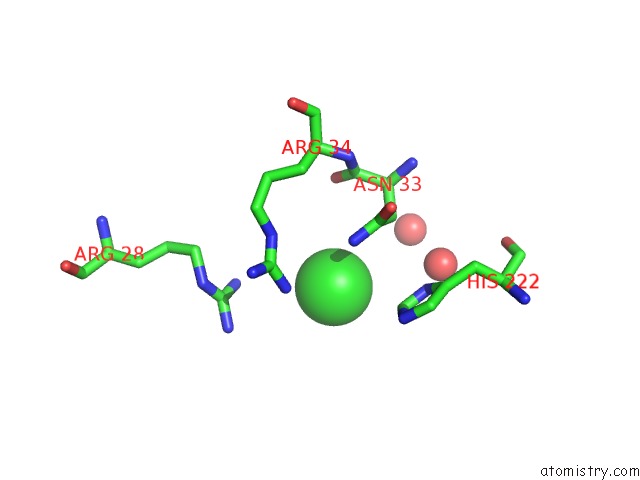
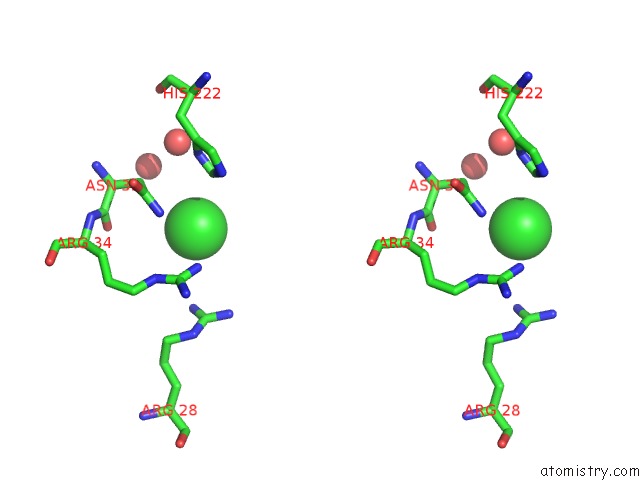
Chlorine binding site 1 out of 3 in 4b6z
Go back to
Chlorine binding site 1 out
of 3 in the Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 1 of Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia within 5.0Å range:
|
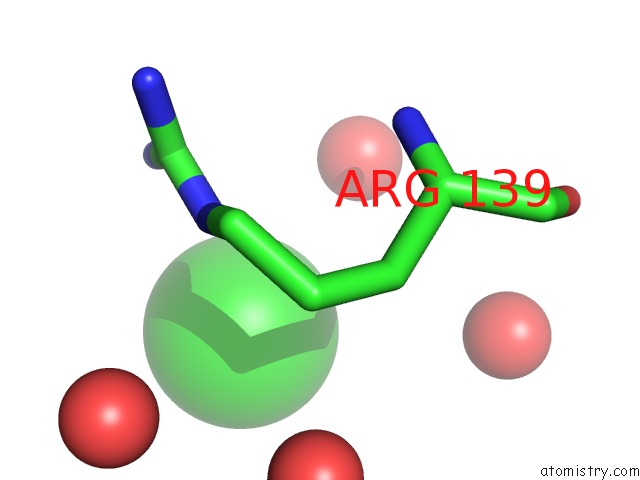
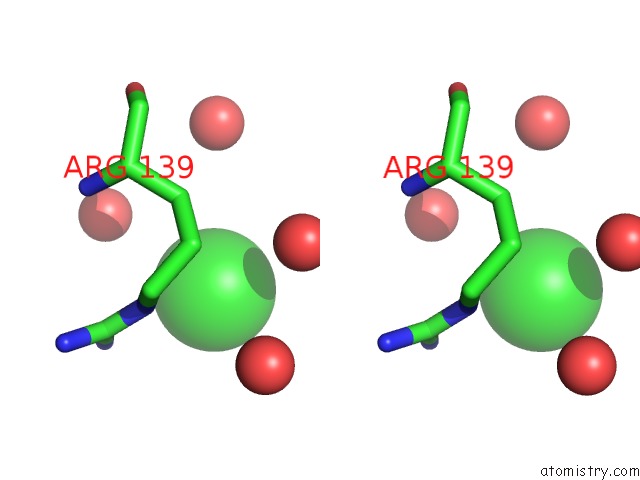
Chlorine binding site 2 out of 3 in 4b6z
Go back to
Chlorine binding site 2 out
of 3 in the Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 2 of Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia within 5.0Å range:
|
Chlorine binding site 3 out of 3 in 4b6z
Go back to
Chlorine binding site 3 out
of 3 in the Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia
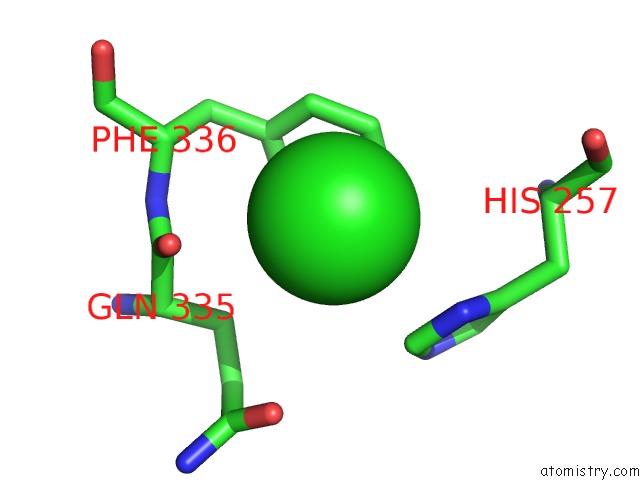
Mono view
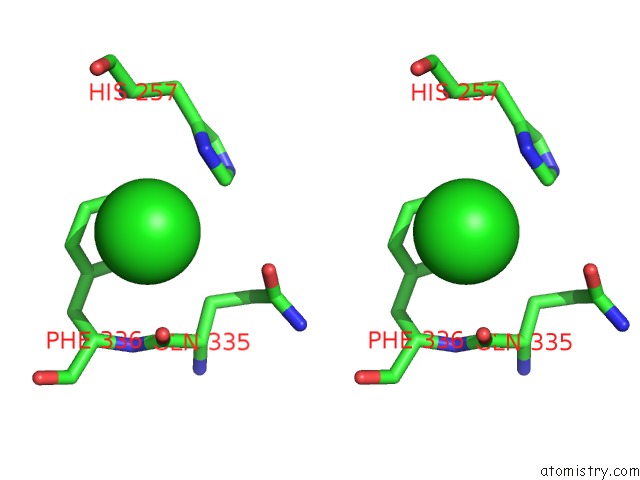
Stereo pair view

Mono view

Stereo pair view
A full contact list of Chlorine with other atoms in the Cl binding
site number 3 of Crystal Structure of Metallo-Carboxypeptidase From Burkholderia Cenocepacia within 5.0Å range:
|
Reference:
V.Rimsa,
T.C.Eadsforth,
R.P.Joosten,
W.N.Hunter.
High-Resolution Structure of the M14-Type Cytosolic Carboxypeptidase From Burkholderia Cenocepacia Refined Exploiting PDB_REDO Strategies. Acta Crystallogr.,Sect.D V. 70 279 2014.
ISSN: ISSN 0907-4449
PubMed: 24531462
DOI: 10.1107/S1399004713026801
Page generated: Sun Jul 21 09:45:29 2024
ISSN: ISSN 0907-4449
PubMed: 24531462
DOI: 10.1107/S1399004713026801
Last articles
Cl in 2VLICl in 2VKE
Cl in 2VJZ
Cl in 2VKG
Cl in 2VKF
Cl in 2VJK
Cl in 2VJL
Cl in 2VJO
Cl in 2VJD
Cl in 2VJJ